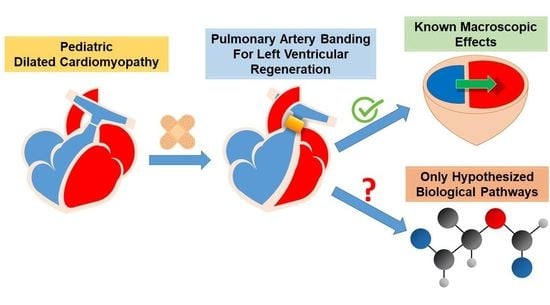
Pulmonary Artery Banding for Dilated Cardiomyopathy in Children: Returning to the Bench from Bedside
Abstract
:1. Introduction
2. Background/The State of the Art
3. Bedside: The Clinical Efficacy of PAB
4. Bedside: Caveats from Clinical Experience
4.1. Age
4.2. Associated Cardiac Defects
4.3. MCS Backup
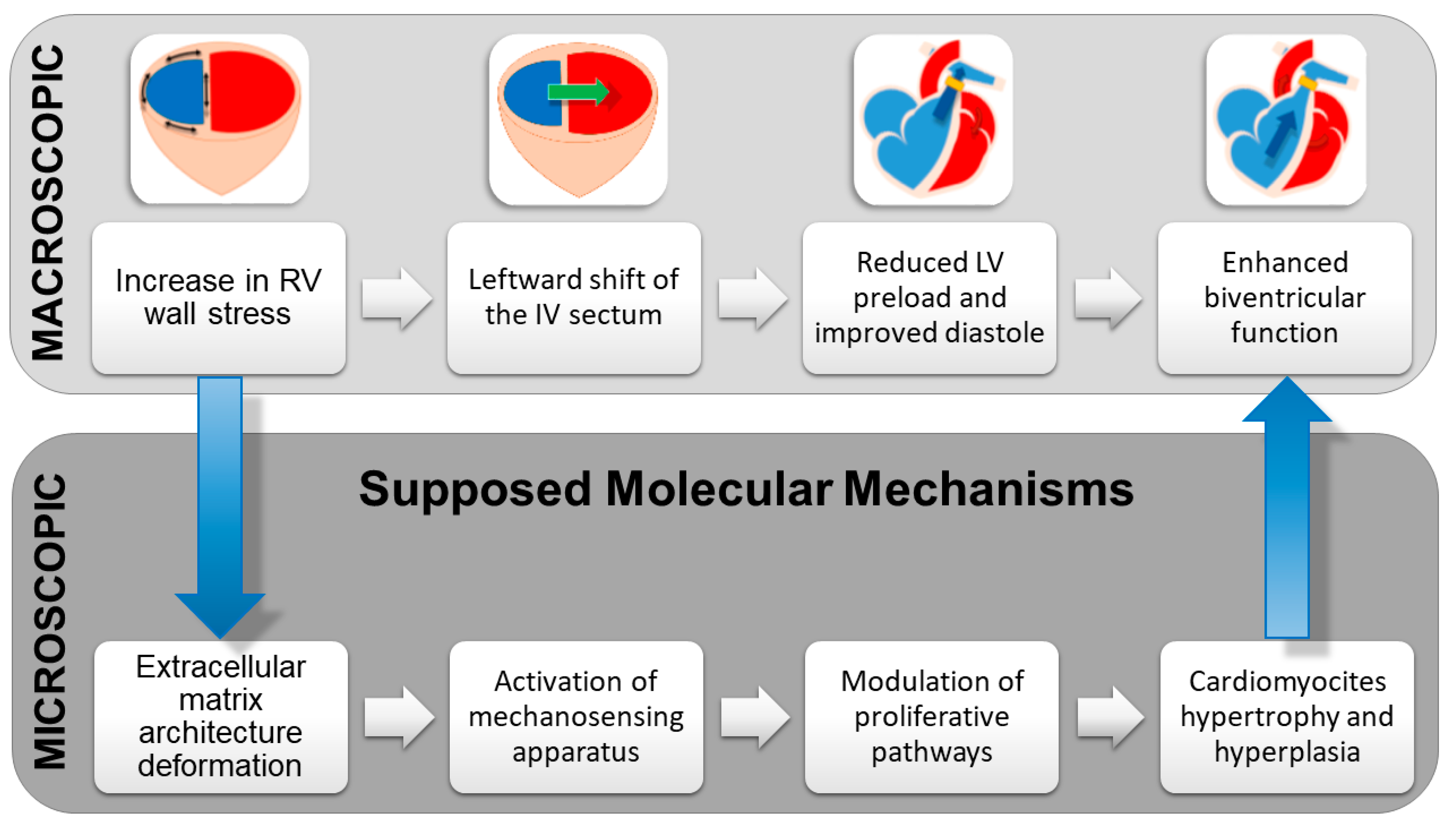
5. Bedside: Functional Changes Induced by PAB
- Increase in RV wall stress, which translates into RV hypertrophy and enhanced contractility due to the Anrep effect.
- Leftward shift of the interventricular septum that contributes to LV reshaping and restoration of an ellipsoidal rather than spherical shape.
- Reduction in LV preload and end-diastolic pressure, with subsequent optimization of the Frank–Starling curve and LV diastolic function.
- Decrease in LV dilatation during the whole cardiac cycle.
- Better left atrial–ventricular coupling and reduction in the severity of mitral regurgitation.
- Enhanced LV contractility.
- Reestablishment of biventricular synchrony.
6. Bench: A Step back to Move Forward
Limitations
7. Conclusion: Stalemate or Turning Point?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossano, J.W.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D.J.; Khush, K.K.; Kucheryavaya, A.Y.; Toll, A.E.; Levvey, B.J.; Meiser, B.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-first pediatric heart transplantation report-2018; Focus theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.E.; George, A.T.; Jaecklin, T.; Lochlainn, E.N.; Thakur, L.; Agrawal, R.; Solar-Yohay, S.; Chen, F.; Rossano, J.W.; Severin, T.; et al. Systematic Literature Review on the Incidence and Prevalence of Heart Failure in Children and Adolescents. Pediatr. Cardiol. 2018, 39, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Akira, M.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Kirk, R.; Dipchand, A.I.; Davies, R.R.; Miera, O.; Chapman, G.; Conway, J.; Denfield, S.; Gossett, J.G.; Johnson, J.; McCulloch, M.; et al. ISHLT consensus statement on donor organ acceptability and management in pediatric heart transplantation. J. Heart Lung Transplant. 2020, 39, 331–341. [Google Scholar] [CrossRef]
- de By, T.M.M.H.; Antonides, C.F.J.; Schweiger, M.; Sliwka, J.; Davies, B.; Berger, F.; Hubler, M.; Ozbaran, M.; Maruszewski, B.; Napoleone, C.P.; et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): Second EUROMACS Paediatric (Paedi-EUROMACS) report. Eur. J. Cardio-Thorac. Surg. 2020, 57, 1038–1050. [Google Scholar] [CrossRef]
- Lorts, A.; Conway, J.; Schweiger, M.; Adachi, I.; Amdani, S.; Auerbach, S.R.; Barr, C.; Bleiweis, M.S.; Blume, E.D.; Burstein, D.S.; et al. ISHLT consensus statement for the selection and management of pediatric and congenital heart disease patients on ventricular assist devices Endorsed by the American Heart Association. J. Heart Lung Transplant. 2021, 40, 709–732. [Google Scholar] [CrossRef]
- Thangappan, K.; Zafar, F.; Lorts, A.; Adachi, I.; Rosenthal, D.; Rossano, J.; Maeda, K.; Morales, D.L. MILESTONE: More Than 1200 Children Bridged to Heart Transplantation with Mechanical Circulatory Support. ASAIO J. 2022, 68, 577–583. [Google Scholar] [CrossRef]
- Chen, J.M.; Canter, C.E.; Hsu, D.T.; Kindel, S.J.; Law, Y.M.; McKeever, J.E.; Pahl, E.; Schumacher, K.R. Current Topics and Controversies in Pediatric Heart Transplantation: Proceedings of the Pediatric Heart Transplantation Summit 2017. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 575–581. [Google Scholar] [CrossRef]
- Zangwill, S. Five decades of pediatric heart transplantation: Challenges overcome, challenges remaining. Curr. Opin. Cardiol. 2017, 32, 69–77. [Google Scholar] [CrossRef]
- Zafar, F.; Conway, J.; Bleiweis, M.S.; Al-Aklabi, M.; Ameduri, R.; Barnes, A.; Bearl, D.W.; Buchholz, H.; Church, S.; Do, N.L.; et al. Berlin Heart EXCOR and ACTION post-approval surveillance study report. J. Heart Lung Transplant. 2021, 40, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Chaggar, P.S.; Williams, S.G.; Yonan, N.; Fildes, J.; Venkateswaran, R.; Shaw, S.M. Myocardial recovery with mechanical circulatory support. Eur. J. Heart Fail. 2016, 18, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Delmo, E.M.J.; Javier, M.F.D.M.; Böthig, D.; Rüffer, A.; Cesnjevar, R.; Dandel, M.; Hetzer, R. Heart failure in the young: Insights into myocardial recovery with ventricular assist device support. Cardiovasc. Diagn. Ther. 2021, 11, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar] [CrossRef]
- Adil, A.; Xu, M.; Haykal, S. Recellularization of Bioengineered Scaffolds for Vascular Composite Allotransplantation. Front. Surg. 2022, 9, 843677. [Google Scholar] [CrossRef]
- Pourmasoumi, P.; Moghaddam, A.; Nemati Mahand, S.; Heidari, F.; Salehi Moghaddam, Z.; Arjmand, M.; Kühnert, I.; Kruppke, B.; Wiesmann, H.-P.; Khonakdar, H.A. A review on the recent progress, opportunities, and challenges of 4D printing and bioprinting in regenerative medicine. J. Biomater. Sci. Polym. Ed. 2022, 1–39. [Google Scholar] [CrossRef]
- Ko, T.; Nomura, S. Manipulating Cardiomyocyte Plasticity for Heart Regeneration. Front. Cell Dev. Biol. 2022, 10, 929256. [Google Scholar] [CrossRef]
- Mills, W.R.; Mal, N.; Kiedrowski, M.J.; Unger, R.; Forudi, F.; Popovic, Z.B.; Penn, M.S.; Laurita, K.R. Stem cell therapy enhances electrical viability in myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 304–314. [Google Scholar] [CrossRef]
- Müller-Ehmsen, J.; Krausgrill, B.; Burst, V.; Schenk, K.; Neisen, U.C.; Fries, J.W.U.; Fleischmann, B.K.; Hescheler, J.; Schwinger, R.H.G. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J. Mol. Cell. Cardiol. 2006, 41, 876–884. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Zhang, P.; Zhang, S.; Chen, P.; Ma, K.; Zhou, C. Bone marrow mononuclear cell transplantation into heart elevates the expression of angiogenic factors. Microvasc. Res. 2004, 68, 156–160. [Google Scholar] [CrossRef]
- Kim, Y.-H. Intramyocardial transplantation of circulating CD34+ cells: Source of stem cells for myocardial regeneration. J. Korean Med. Sci. 2003, 18, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, S.; Ohtsuki, S.; Eitoku, T.; Ousaka, D.; Kondo, M.; Kurita, Y.; Hirai, K.; Fukushima, Y.; Baba, K.; Goto, T.; et al. Intracoronary Cardiac Progenitor Cells in Single Ventricle Physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) Randomized Phase 2 Trial. Circ. Res. 2017, 120, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Kereiakes, D.J.; Aguirre, F.; Kowalchuk, G.; Chakravarty, T.; Malliaras, K.; Francis, G.S.; Povsic, T.J.; Schatz, R.; Traverse, J.H.; et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Monabati, A.; Montaseri, M.; Vosough, M.; Hosseini, S.A.; Kojouri, J.; Abdi-Ardekani, A.; Izadpanah, P.; Azarpira, N.; Pouladfar, G.; et al. Transplantation of mesenchymal stem cells for prevention of acute myocardial infarction induced heart failure: Study protocol of a phase III randomized clinical trial (Prevent-TAHA8). Trials 2022, 23, 632. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D.; Veldman, A.; Bartram, U.; Michel-Behnke, I.; Bauer, J.; Akintürk, H. Pulmonary artery banding for idiopathic dilative cardiomyopathy: A novel therapeutic strategy using an old surgical procedure. J. Thorac. Cardiovasc. Surg. 2007, 134, 796–797. [Google Scholar] [CrossRef]
- Koestenberger, M.; Sallmon, H.; Avian, A.; Cantinotti, M.; Gamillscheg, A.; Kurath-Koller, S.; Schweintzger, S.; Hansmann, G. Ventricular-ventricular interaction variables correlate with surrogate variables of clinical outcome in children with pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019854074. [Google Scholar] [CrossRef]
- Friedberg, M.K. Imaging Right-Left Ventricular Interactions. JACC Cardiovasc. Imaging 2018, 11, 755–771. [Google Scholar] [CrossRef]
- Schranz, D.; Rupp, S.; Müller, M.; Schmidt, D.; Bauer, A.; Valeske, K.; Michel-Behnke, I.; Jux, C.; Apitz, C.; Thul, J.; et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: A novel therapeutic strategy before heart transplantation. J. Heart Lung Transplant. 2013, 32, 475–481. [Google Scholar] [CrossRef]
- Schranz, D.; Akintuerk, H.; Bailey, L. Pulmonary Artery Banding for Functional Regeneration of End-Stage Dilated Cardiomyopathy in Young Children: World Network Report. Circulation 2018, 137, 1410–1412. [Google Scholar] [CrossRef]
- Spigel, Z.A.; Razzouk, A.; Nigro, J.J.; Karamlou, T.B.; Kavarana, M.N.; Roeser, M.E.; Adachi, I. Pulmonary Artery Banding for Children with Dilated Cardiomyopathy: US Experience. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2020, 23, 69–76. [Google Scholar] [CrossRef]
- Ponzoni, M.; Frigo, A.C.; Castaldi, B.; Cerutti, A.; Di Salvo, G.; Vida, V.L.; Padalino, M.A. Surgical strategies for the management of end-stage heart failure in infants and children: A 15-year experience with a patient-tailored approach. Artif. Organs 2021, 45, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.L.S.; Adachi, I.; Peng, D.M.; Sinha, P.; Lorts, A.; Fields, K.; Conway, J.; Louis, J.D.S.; Cantor, R.; Koehl, D.; et al. Fourth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report. Ann. Thorac. Surg. 2020, 110, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Rossano, J.W.; Singh, T.P.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.J.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report—2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1028–1041. [Google Scholar] [CrossRef]
- Rossano, J.W.; Kantor, P.F.; Shaddy, R.E.; Shi, L.; Wilkinson, J.D.; Jefferies, J.L.; Czachor, J.D.; Razoky, H.; Wirtz, H.S.; Depre, C.; et al. Elevated Heart Rate and Survival in Children with Dilated Cardiomyopathy: A Multicenter Study from the Pediatric Cardiomyopathy Registry. J. Am. Heart Assoc. 2020, 9, e015916. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Aquaro, G.D.; Pingitore, A.; Masci, P.G.; Lombardi, M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur. J. Heart Fail. 2011, 13, 170–176. [Google Scholar] [CrossRef]
- Sharma, S.; Mishra, R.; Bigham, G.E.; Wehman, B.; Khan, M.M.; Xu, H.; Saha, P.; Goo, Y.A.; Datla, S.R.; Chen, L.; et al. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ. Res. 2017, 120, 816–834. [Google Scholar] [CrossRef]
- Traister, A.; Patel, R.; Huang, A.; Patel, S.; Plakhotnik, J.; Lee, J.E.; Medina, M.G.; Welsh, C.; Ruparel, P.; Zhang, L.; et al. Cardiac regenerative capacity is age- and disease-dependent in childhood heart disease. PLoS ONE 2018, 13, e0200342. [Google Scholar]
- Latus, H.; Gummel, K.; Rupp, S.; Mueller, M.; Jux, C.; Kerst, G.; Akintuerk, H.; Bauer, J.; Schranz, D.; Apitz, C. Cardiovascular magnetic resonance assessment of ventricular function and myocardial scarring before and early after repair of anomalous left coronary artery from the pulmonary artery. J. Cardiovasc. Magn. Reson. 2014, 16, 3. [Google Scholar] [CrossRef]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.-I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart after Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, Y.; Dong, S.; Du, C.; Yan, J. Two-Stage Arterial Switch for Transposition of the Great Vessels in Older Children. Ann. Thorac. Surg. 2022, 114, 193–200. [Google Scholar] [CrossRef]
- Boutin, C.; Jonas, R.A.; Sanders, S.P.; Wernovsky, G.; Mone, S.M.; Colan, S.D. Rapid two-stage arterial switch operation. Acquisition of left ventricular mass after pulmonary artery banding in infants with transposition of the great arteries. Circulation 1994, 90, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedmera, D.; Reckova, M.; DeAlmeida, A.; Coppen, S.R.; Kubalak, S.W.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 274, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Zacchigna, S.; Martinelli, V.; Moimas, S.; Colliva, A.; Anzini, M.; Nordio, A.; Costa, A.; Rehman, M.; Vodret, S.; Pierro, C.; et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat. Commun. 2018, 9, 2432. [Google Scholar] [CrossRef]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef]
- Canseco, D.C.; Kimura, W.; Garg, S.; Mukherjee, S.; Bhattacharya, S.; Abdisalaam, S.; Das, S.; Asaithamby, A.; Mammen, P.P.; Sadek, H.A. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 2015, 65, 892–900. [Google Scholar] [CrossRef]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo signaling impedes adult heart regeneration. Development 2013, 140, 4683–4690. [Google Scholar] [CrossRef]
- Tsai, C.-R.; Martin, J.F. Hippo signaling in cardiac fibroblasts during development, tissue repair, and fibrosis. Curr. Top. Dev. Biol. 2022, 149, 91–121. [Google Scholar]
- Zhang, W.; Li, Q.-Q.; Gao, H.-Y.; Wang, Y.-C.; Cheng, M.; Wang, Y.-X. The regulation of yes-associated protein/transcriptional coactivator with PDZ-binding motif and their roles in vascular endothelium. Front. Cardiovasc. Med. 2022, 9, 925254. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Asghari, S.; Mansouri, P.; Alemi, F.; Majidinia, M.; Mahmoodpoor, A.; Yousefi, B. The Roles of Signaling Pathways in Cardiac Regeneration. Curr. Med. Chem. 2022, 29, 2142–2166. [Google Scholar] [CrossRef]
- Morikawa, Y.; Heallen, T.; Leach, J.; Xiao, Y.; Martin, J.F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 2017, 547, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Schranz, D.; Recla, S.; Malcic, I.; Kerst, G.; Mini, N.; Akintuerk, H. Pulmonary artery banding in dilative cardiomyopathy of young children: Review and protocol based on the current knowledge. Transl. Pediatr. 2019, 8, 151–160. [Google Scholar] [CrossRef]
- Winlaw, D.S.; McGuirk, S.P.; Balmer, C.; Langley, S.M.; Griselli, M.; Stümper, O.; De Giovanni, J.V.; Wright, J.G.; Thorne, S.; Barron, D.J.; et al. Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a view to anatomic biventricular repair. Circulation 2005, 111, 405–411. [Google Scholar] [CrossRef]
- Myers, P.O.; del Nido, P.J.; Geva, T.; Bautista-Hernandez, V.; Chen, P.; Mayer, J.E.J.; Emani, S.M. Impact of age and duration of banding on left ventricular preparation before anatomic repair for congenitally corrected transposition of the great arteries. Ann. Thorac. Surg. 2013, 96, 603–610. [Google Scholar] [CrossRef]
- Ibrahimiye, A.N.; Mainwaring, R.D.; Patrick, W.L.; Downey, L.; Yarlagadda, V.; Hanley, F.L. Left Ventricular Retraining and Double Switch in Patients with Congenitally Corrected Transposition of the Great Arteries. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 203–209. [Google Scholar] [CrossRef]
- Trescher, K.; Bauer, M.; Dietl, W.; Hallström, S.; Wick, N.; Wolfsberger, M.; Ullrich, R.; Jurgens, G.; Wolner, E.; Podesser, B.K. Improved myocardial protection in the failing heart by selective endothelin-A receptor blockade. J. Thorac. Cardiovasc. Surg. 2009, 137, 1005–1011.e1. [Google Scholar] [CrossRef]
- Ansari, A. Anatomy and clinical significance of ventricular Thebesian veins. Clin. Anat. 2001, 14, 102–110. [Google Scholar] [CrossRef]
- Crystal, G.J.; Pagel, P.S. Right Ventricular Perfusion: Physiology and Clinical Implications. Anesthesiology 2018, 128, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Mankad, P.S.; Yacoub, M.H. Systolic and diastolic function of both ventricles after prolonged cardioplegic arrest. Ann. Thorac. Surg. 1993, 55, 933–939. [Google Scholar] [CrossRef]
- Abdul-Ghani, S.; Skeffington, K.L.; Kim, M.; Moscarelli, M.; Lewis, P.A.; Heesom, K.; Fiorentino, F.; Emanueli, C.; Reeves, B.C.; Punjabi, P.P.; et al. Effect of cardioplegic arrest and reperfusion on left and right ventricular proteome/phosphoproteome in patients undergoing surgery for coronary or aortic valve disease. Int. J. Mol. Med. 2022, 49, 77. [Google Scholar] [CrossRef] [PubMed]
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 708–718. [Google Scholar] [CrossRef]
- Murphy, C.O.; Pan-Chih Gott, J.P.; Guyton, R.A. Microvascular reactivity after crystalloid, cold blood, and warm blood cardioplegic arrest. Ann. Thorac. Surg. 1995, 60, 1021–1027. [Google Scholar] [CrossRef]
- Kortekaas, K.A.; Lindeman, J.H.; Versteegh, M.I.; van Beelen, E.; Kleemann, R.; Klautz, R.J. Heart failure determines the myocardial inflammatory response to injury. Eur. J. Heart Fail. 2013, 15, 400–407. [Google Scholar] [CrossRef]
- Morales, D.L.S.; Rossano, J.W.; VanderPluym, C.; Lorts, A.; Cantor, R.; St. Louis, J.D.; Koeh, D.; Sutcliffe, D.L.; Adachi, I.; Kirklin, J.K.; et al. Third Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report: Preimplant Characteristics and Outcomes. Ann. Thorac. Surg. 2019, 107, 993–1004. [Google Scholar] [CrossRef]
- Di Candia, A.; Castaldi, B.; Bordin, G.; Cerutti, A.; Reffo, E.; Biffanti, R.; Di Salvo, G.; Vida, V.L.; Padalino, M. A Pulmonary Artery Banding for Ventricular Rehabilitation in Infants with Dilated Cardiomyopathy: Early Results in a Single-Center Experience. Front. Pediatr. 2020, 8, 347. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, E.S.; Kwon, B.S.; Park, C.S.; Cha, S.G.; Baek, J.S.; Yu, J.J.; Kim, Y.-H.; Yun, T.-J. Development of Cardiac Events and Functional Recovery Prediction Models for Pediatric Dilated Cardiomyopathy. Front. Pediatr. 2021, 9, 736872. [Google Scholar]
- Wang, P.-Y.; Tseng, W.-C.; Fu, C.-M.; Wu, M.-H.; Wang, J.-K.; Chen, Y.-S.; Chou, N.-K.; Wang, S.-S.; Chiu, S.-N.; Lin, M.-T.; et al. Long-Term Outcomes and Prognosticators of Pediatric Primary Dilated Cardiomyopathy in an Asian Cohort. Front. Pediatr. 2021, 9, 771283. [Google Scholar] [CrossRef]
- Latus, H.; Hachmann, P.; Gummel, K.; Recla, S.; Voges, I.; Mueller, M.; Bauer, J.; Yerebakan, C.; Akintuerk, H.; Apitz, C.; et al. Biventricular response to pulmonary artery banding in children with dilated cardiomyopathy. J. Heart Lung Transplant. 2016, 35, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Chen, Y.-S.; Lin, M.-T.; Chen, C.-A. Improved Left Ventricular Strain and Dyssynchrony after Pulmonary Artery Banding in an Infant with End-Stage Dilated Cardiomyopathy: Insights from Three-Dimensional Speckle Tracking. Pediatr. Cardiol. 2019, 40, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Boltze, J.; Elmontaser, H.; Yoruker, U.; Latus, H.; Khalil, M.; Ostermayer, S.; Steinbrenner, B.; Apitz, C.; Schneider, M.; et al. Effects of pulmonary artery banding in doxorubicin-induced left ventricular cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2019, 157, 2416–2428.e4. [Google Scholar] [CrossRef]
- Borenstein, N.; Bruneval, P.; Behr, L.; Laborde, F.; Montarras, D.; Daurès, J.P.; Derumeaux, G.; Pouchelon, J.-L.; Chetboul, V. An ovine model of chronic heart failure: Echocardiographic and tissue Doppler imaging characterization. J. Card. Surg. 2006, 21, 50–56. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.L.; Romano, M.M.D.; Campos Pulici, E.C.; Carvalho, E.E.V.; de Souza, F.R.; Tanaka, D.M.; Maciel, B.C.; Salgado, H.C.; Fazan, R., Jr.; Rossi, M.A.; et al. Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: A comparison of functional and histopathological changes. Exp. Toxicol. Pathol. 2017, 69, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Boczar, K.E.; Aseyev, O.; Sulpher, J.; Johnson, C.; Burwash, I.G.; Turek, M.; Dent, S.; Dwivedi, G. Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res. Pract. 2016, 3, 79–84. [Google Scholar] [CrossRef]
- Rahmanifard, M.; Vessal, M.; Noorafshan, A.; Karbalay-Doust, S.; Naseh, M. The Protective Effects of Coenzyme Q10 and Lisinopril against Doxorubicin-Induced Cardiotoxicity in Rats: A Stereological and Electrocardiogram Study. Cardiovasc. Toxicol. 2021, 21, 936–946. [Google Scholar] [CrossRef]
- Yu, H.-L.; Hwang, S.-P.L. Zebrafish integrin a3b is required for cardiac contractility and cardiomyocyte proliferation. Biochem. Biophys. Res. Commun. 2022, 595, 89–95. [Google Scholar] [CrossRef]
- Chakraborty, S.; Njah, K.; Pobbati, A.V.; Lim, Y.B.; Raju, A.; Lakshmanan, M.; Tergaonkar, V.; Lim, C.T.; Hong, W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017, 18, 2464–2479. [Google Scholar] [CrossRef]
- Zhou, J. An emerging role for Hippo-YAP signaling in cardiovascular development. J. Biomed. Res. 2014, 28, 251–254. [Google Scholar]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, S.; Montecucco, F.; Kolijn, D.; Pedicino, D.; Akhmedov, A.; Mohammed, S.A.; Herwig, M.; Gorica, E.; Szabó, P.L.; Weber, L.; et al. Methylation of the Hippo effector YAP by the methyltransferase SETD7 drives myocardial ischemic injury: A translational study. Cardiovasc. Res. 2022, cvac102. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, F.; Sorrentino, A.; van der Reest, J.; Yang, P.; Waldeck-Weiermair, M.; Steinhorn, B.; Eroglu, E.; Saeedi Saravi, S.S.; Yu, P.; Haigis, M.; et al. Metabolomic and transcriptomic signatures of chemogenetic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H451–H465. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.J.; Dalinghaus, M.; Lankhuizen, I.M.; Bezstarosti, K.; Dekkers, D.H.W.; Duncker, D.J.; Helbing, W.A.; Lamers, J.M.J. Proteomic changes in the pressure overloaded right ventricle after 6 weeks in young rats: Correlations with the degree of hypertrophy. Proteomics 2005, 5, 2519–2530. [Google Scholar] [CrossRef]
- Faber, M.J.; Dalinghaus, M.; Lankhuizen, I.M.; Bezstarosti, K.; Verhoeven, A.J.M.; Duncker, D.J.; Helbing, W.A.; Lamers, J.M.J. Time dependent changes in cytoplasmic proteins of the right ventricle during prolonged pressure overload. J. Mol. Cell. Cardiol. 2007, 43, 197–209. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Wu, M.; Song, J.; Zhang, M.; Duan, Y.; Jiang, K.; Zhou, X.; Zhang, Y. RNA-sequencing analysis of gene expression in a rat model of acute right heart failure. Pulm. Circ. 2020, 10, 2045894019879396. [Google Scholar] [CrossRef]
- Friehs, I.; Cowan, D.B.; Choi, Y.-H.; Black, K.M.; Barnett, R.; Bhasin, M.K.; Daly, C.; Dillon, S.J.; Libermann, T.A.; McGowan, F.X.; et al. Pressure-overload hypertrophy of the developing heart reveals activation of divergent gene and protein pathways in the left and right ventricular myocardium. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H697–H708. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponzoni, M.; Castaldi, B.; Padalino, M.A. Pulmonary Artery Banding for Dilated Cardiomyopathy in Children: Returning to the Bench from Bedside. Children 2022, 9, 1392. https://doi.org/10.3390/children9091392
Ponzoni M, Castaldi B, Padalino MA. Pulmonary Artery Banding for Dilated Cardiomyopathy in Children: Returning to the Bench from Bedside. Children. 2022; 9(9):1392. https://doi.org/10.3390/children9091392
Chicago/Turabian StylePonzoni, Matteo, Biagio Castaldi, and Massimo A. Padalino. 2022. "Pulmonary Artery Banding for Dilated Cardiomyopathy in Children: Returning to the Bench from Bedside" Children 9, no. 9: 1392. https://doi.org/10.3390/children9091392
APA StylePonzoni, M., Castaldi, B., & Padalino, M. A. (2022). Pulmonary Artery Banding for Dilated Cardiomyopathy in Children: Returning to the Bench from Bedside. Children, 9(9), 1392. https://doi.org/10.3390/children9091392