Clinical Accuracy of Non-Contact Forehead Infrared Thermometer Measurement in Children: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements
2.3. Data Collection/Procedure
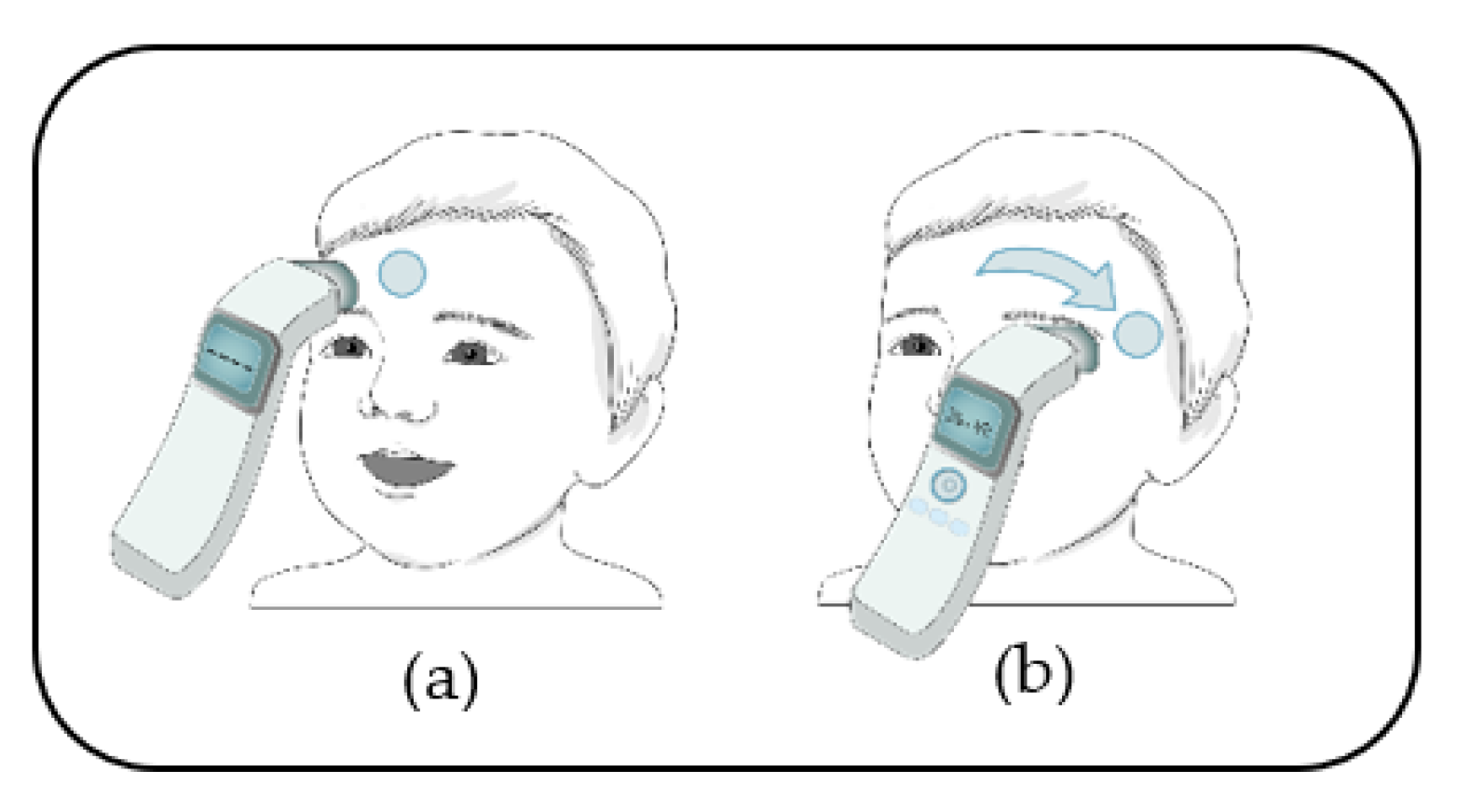
2.3.1. Measurement Using the NCFIT
2.3.2. Measurement Using the IRTT
2.3.3. Readings across Instruments
2.4. Statistical Analysis
2.5. Sample Size
3. Results
3.1. Participant Characteristics
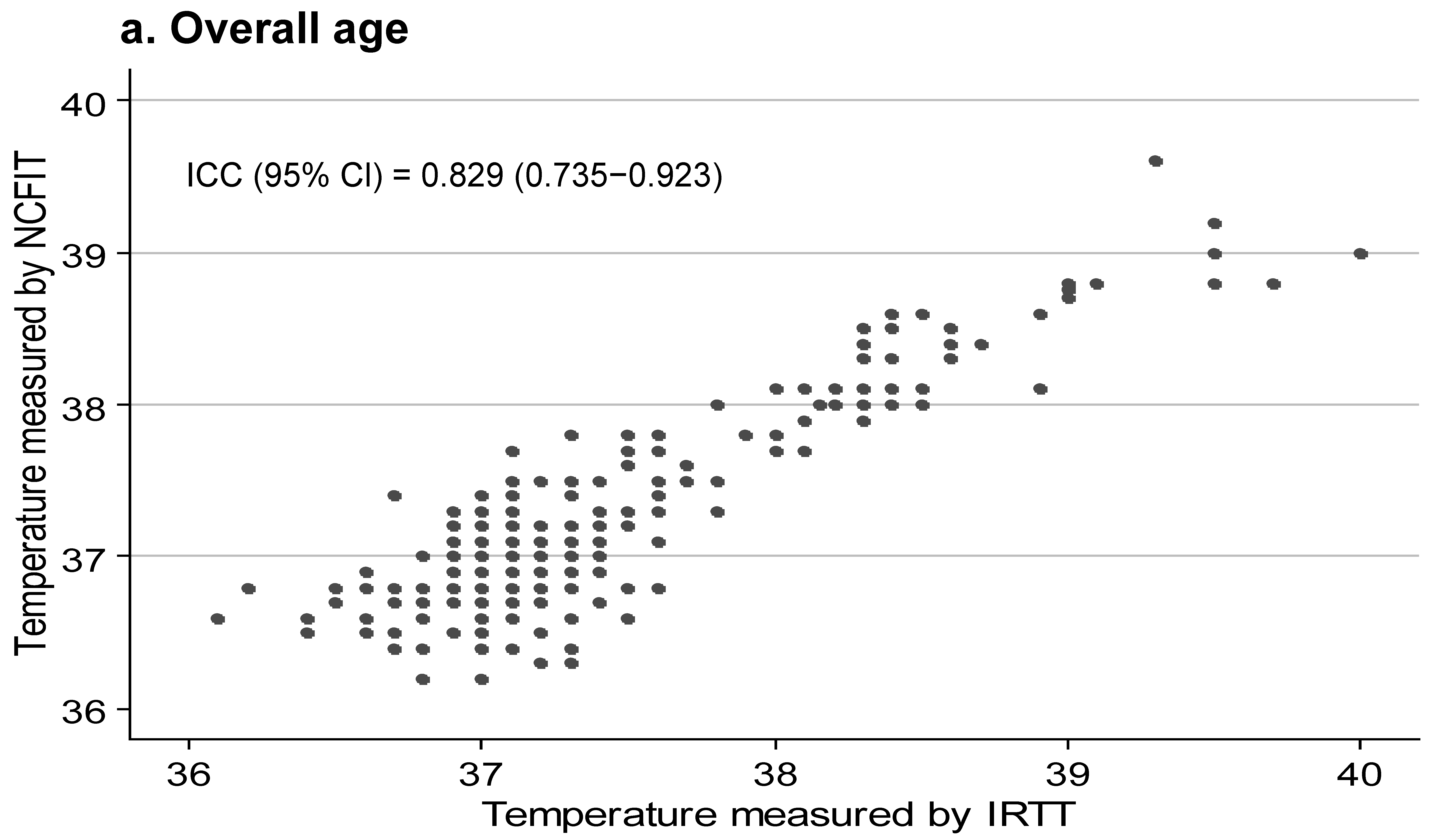
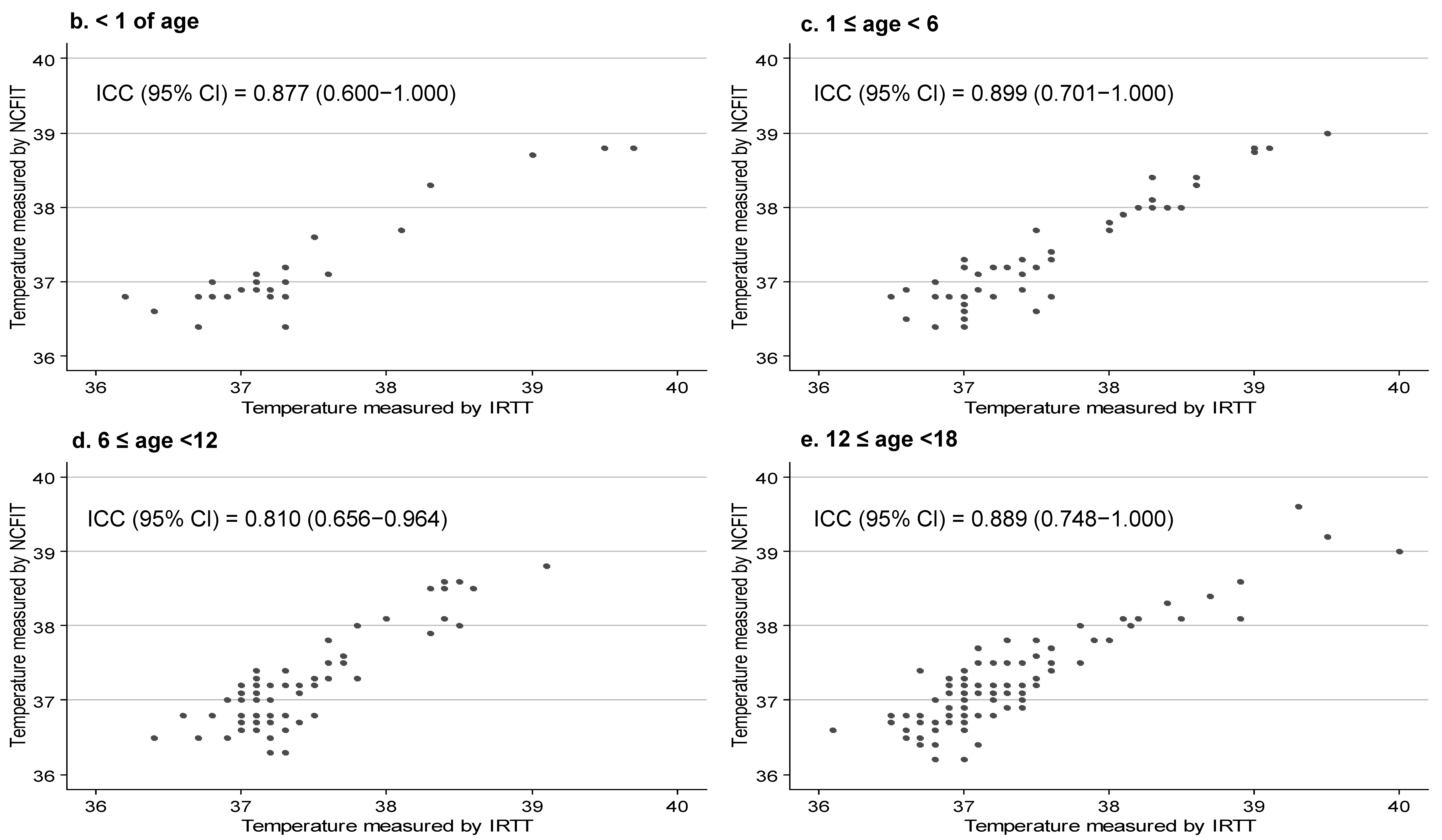
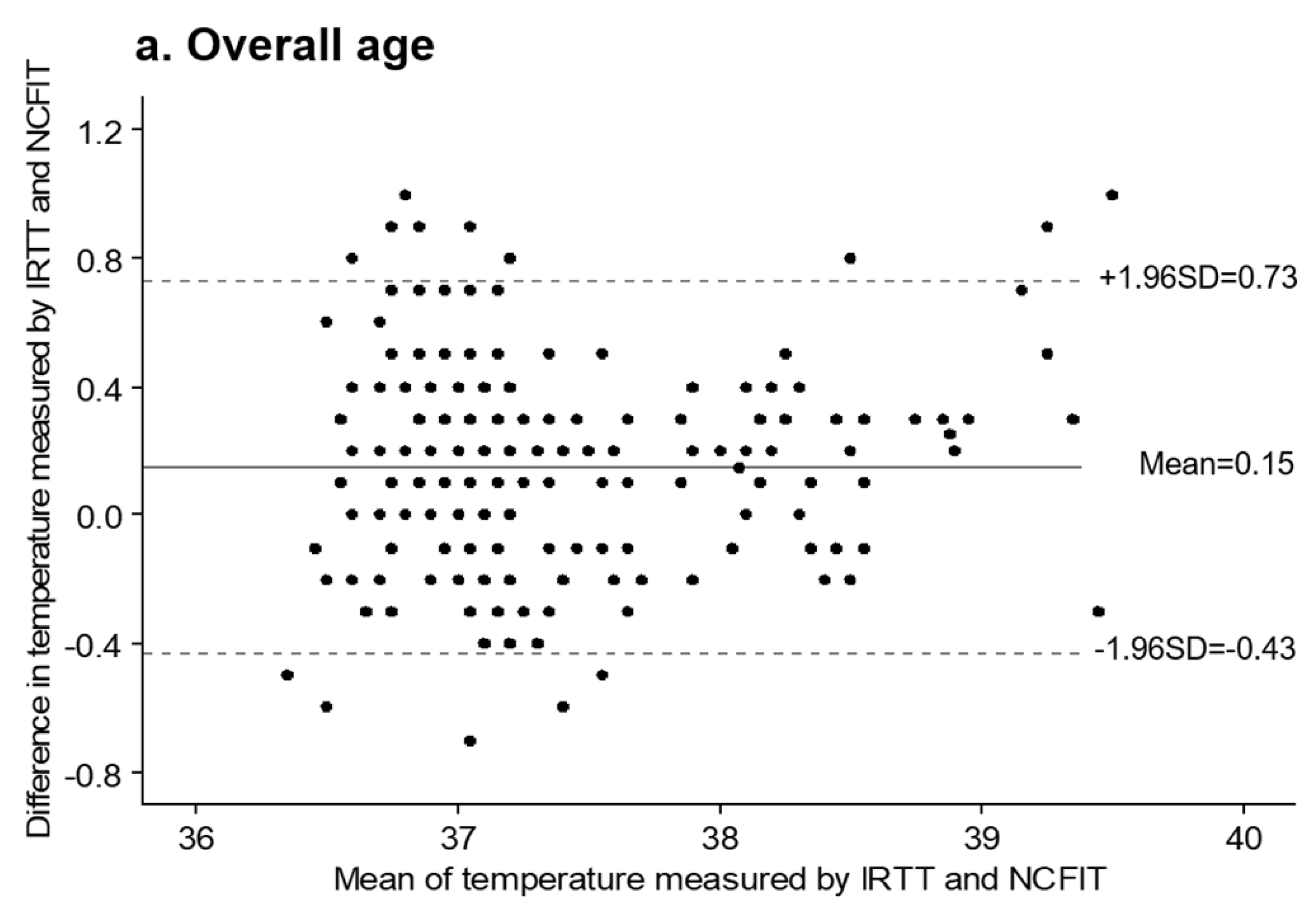
3.2. Agreement between NCFIT and IRTT
3.3. Validity of Fever Detection Based on 38.0 °C of IRTT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moran, J.L.; Peter, J.V.; Solomon, P.J.; Grealy, B.; Smith, T.; Ashforth, W.; Wake, M.; Peake, S.L.; Peisach, A.R. Tympanic temperature measurements: Are they reliable in the critically ill? A clinical study of measures of agreement. Crit. Care Med. 2007, 35, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.H.; Carrigan, J.D.; Solomon, D.M.; Tart, R.C. Temporal Artery Thermometry to Detect Pediatric Fever. Clin. Nurs. Res. 2015, 24, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.L.; Rinaldi, J.E.; Hariharan, P.; Casamento, J.P.; Baek, S.; Seay, N.; Vesnovsky, O.; Topoleski, T. Clinical evaluation of non-contact infrared thermometers. Sci. Rep. 2021, 11, 22079. [Google Scholar] [CrossRef] [PubMed]
- Sollai, S.; Dani, C.; Berti, E.; Fancelli, C.; Galli, L.; de Martino, M.; Chiappini, E. Performance of a non-contact infrared thermometer in healthy newborns. BMJ Open. 2016, 6, e008695. [Google Scholar] [CrossRef]
- ISO 80601-2-56; Particular Requirements for Basic Safety and Essential Performance of Clinical Thermometers for Body Temperature Measurement. ISO: Geneva, Switzerland, 2017.
- Chiappini, E.; Venturini, E.; Remaschi, G.; Principi, N.; Longhi, R.; Tovo, P.A.; Becherucci, P.; Bonsignori, F.; Esposito, S.; Festini, F.; et al. 2016 Update of the Italian Pediatric Society Guidelines for Management of Fever in Children. J. Pediatr. 2017, 180, 177–183. [Google Scholar] [CrossRef]
- Sehgal, A.; Debey, N.K.; Jyothi, M.C.; Jain, S. Comparison of tympanic and rectal temperature in febrile patients. Indian J. Pediatr. 2002, 69, 305–308. [Google Scholar] [CrossRef]
- Hayward, G.; Verbakel, J.Y.; Ismail, F.A.; Edwards, G.; Wang, K.; Fleming, S.; Holtman, G.A.; Golgowska, M.; Morris, E.; Curits, K.; et al. Non-contact infrared versus axillary and tympanic thermometers in childrne attending primary care: A mixed-methods study of accuracy and acceptabiltiy. Br. J. Gen. Prac. 2020, 70, e236–e244. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, L.Y.; Li, H.X. Diagnostic test accuracy of new generation tympanic thermometry in children under different cutoffs: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 210. [Google Scholar] [CrossRef]
- FDA. Clinical Electronic Thermometer. Product Classification. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm?ID=FLL (accessed on 22 August 2022).
- Wang, K.; Gill, P.; Wolstenholme, J.; Price, C.P.; Heneghan, C.; Thompson, M.; Pluddemann, A. Non-contact infrared thermometers for measuring temperature in children: Primary care diagnostic technology update. Br. J. Gen. Pract. 2014, 64, e681–e683. [Google Scholar] [CrossRef]
- ASTM E1965-98; Standard Specification for Infrared Thermometers for Intermittent Determination of Patient Temperature. ASTM International: West Conshohocken, PA, USA, 2016.
- Mogensen, C.B.; Wittenhoff, L.; Fruerhøj, G.; Hansen, S. Forehead or ear temperature measurement cannot replace rectal measurements, except for screening purposes. BMC Pediatr. 2018, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Kocoglu, H.; Goksu, S.; Isik, M.; Akturk, Z.; Bayazit, Y.A. Infrared tympanic thermometer can accurately measure the body temperature in children in an emergency room setting. Int. J. Pediatr. Otorhinolaryngol. 2002, 65, 39–43. [Google Scholar] [CrossRef]
- Erickson, R.S.; Kirklin, S.K. Comparison of ear-based, bladder, oral, and axillary methods for core temperature measurement. Crit. Care Med. 1993, 21, 1528–1534. [Google Scholar] [CrossRef]
- Fortuna, E.L.; Carney, M.M.; Macy, M.; Stanley, R.M.; Younger, J.G.; Bradin, S.A. Accuracy of non-contact infrared thermometry versus rectal thermometry in young children evaluated in the emergency department for fever. J. Emerg. Nurs. 2010, 36, 101–104. [Google Scholar] [CrossRef]
- Aggarwal, N.; Garg, M.; Dwarakanathan, V.; Gautam, N.; Kumar, S.S.; Jadon, R.S.; Gupta, M.; Ray, A. Diagnostic accuracy of non-contact infrared thermometers and thermal scanners: A systematic review and meta-analysis. J. Travel Med. 2020, 27, taa193. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.; Kang, C. Systematic review and meta-analysis of diagnostic accuracy of thermometer when identifying fever in children. J. Korean Acad. Nurs. 2013, 43, 746–759. [Google Scholar] [CrossRef]
- Teran, C.G.; Torrez-Llanos, J.; Teran-Miranda, T.E.; Balderrama, C.; Shah, N.S.; Villarroel, P. Clinical accuracy of a non-contact infrared skin thermometer in paediatric practice. Child Care Health Dev. 2012, 38, 471–476. [Google Scholar] [CrossRef]
- Osio, C.E.; Carnelli, V. Comparative study of body temperature measured with a non-contact infrared thermometer versus conventional devices. The first Italian study on 90 pediatric patients. Minerva Pediatr. 2007, 59, 327–336. [Google Scholar]
- Sethi, A.; Patel, D.; Nimbalkar, A.; Phatak, A.; Nimbalkar, S. Comparison of forehead infrared thermometry with axillary digital thermometry in neonates. Indian Pediatr. 2013, 50, 1153–1154. [Google Scholar] [CrossRef]
- Paes, B.F.; Vermeulen, K.; Brohet, R.M.; van der Ploeg, T.; de Winter, J.P. Accuracy of tympanic and infrared skin thermometers in children. Arch. Dis. Child. 2010, 95, 974–978. [Google Scholar] [CrossRef]
- Berkosy, E.A.; Anıl, M.; Bıcılıoğlu, Y.; Gökalp, G.; Bal, A. Original Article Comparison of infrared tympanic, non-contact infrared skin, and axillary thermometer to rectal temperature measurements in a pediatric emergency observation unit. Int. J. Clin. Exp. Med. 2018, 11, 567–573. [Google Scholar]
- El-Radhi, A.S.; Barry, W. Thermometry in paediatric practice. Arch. Dis. Child. 2006, 91, 351–356. [Google Scholar] [CrossRef]
- FDA. Non-Contact Infrared Thermometer. Available online: https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/non-contact-infrared-thermometers (accessed on 22 June 2022).
- Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliability, and pitfalls and research and practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Basak, T.; Aciksoz, S.; Tosun, B.; Akyuz, A.; Acikel, C. Comparison of three different thermometers in evaluating the body temperature of healthy young adult individuals. Int. J. Nurs. Pract. 2013, 19, 471–478. [Google Scholar] [CrossRef]
- Kong, K.A. Statistical Methods: Reliability assessment and method comparison. Ewha Med. J. 2017, 40, 9–16. [Google Scholar] [CrossRef]
- David, M.; Campbell, M.J.; Tan, S.B.; Tan, S.H. Sample Size Tables for Clinical Studies, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; p. 194. [Google Scholar]
- Vancouver: Arifin WN. Sample Size Calculator (Version 2.0) [Internet]. 2017. [Revised 2017 October]. Available online: https://wnarifin.github.io (accessed on 2 April 2022).
- Bruel, A.V.D.; Verbakel, J.; Wang, K.; Fleming, S.; Holtman, G.; Glogowska, M.; Morris, E.; Edwards, G.; Abakar Ismail, F.; Curtis, K.; et al. Non-contact infrared thermometers compared with current approaches in primary care for children aged 5 years and under: A method comparison study. Health Technol. Assess. 2020, 24, 1–28. [Google Scholar] [CrossRef]
- Hamilton, P.A.; Marcos, L.S.; Secic, M. Performance of infrared ear and forehead thermometers: A comparative study in 205 febrile and afebrile children. J. Clin. Nurs. 2013, 22, 2509–2518. [Google Scholar] [CrossRef]
- Child, C.; Harrison., R.; Hodkinson, C. Tympanic membrane temperature as a measure of core temperature. Arch. Dis. Child. 1999, 80, 262–266. [Google Scholar] [CrossRef]
- Gerke, O. Reporting standards for a Bland-Altman agreement analysis: A review of mythological reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef]
- Ng, D.K.; Chan, C.; Lee, R.S.; Leung, L.C. Non-contact infrared thermometry temperature measurement for screening fever in children. Ann. Trop Paediatr. 2005, 25, 267–275. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, A.; Chen, C. Investigation of the impact of infrared sensors on core body temperature monitoring by comparing measurements sites. Sensors 2020, 20, 2885. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Chen, C. Evaluation of performance and uncertainty of infrared tympanic thermometer. Sensors 2010, 10, 3073–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C. Evaluation of measurement uncertainty for thermometers with calibration equations. Accredit. Qual. Assur. 2006, 11, 75–82. [Google Scholar] [CrossRef]
- Hong Kong Medical Device Division. Proper Use of Thermometers to Measure Body Temperature. 2018. Available online: https://www.mdd.gov.hk/english/emp/emp_gp/files/thermometer_eng.pdf (accessed on 22 July 2022).
- Lai, F.; Li, X.; Luo, Y.; Wang, X.; Huang, X.; Zhang, J.; Peng, J.; Wang, Q.; Fan, L.; Li, W.; et al. Reliability of non-contact infrared thermometers for fever screening under COVID-19. Risk Manag. Health Policy 2022, 15, 447–456. [Google Scholar] [CrossRef]

| Overall (n = 255) | Age < 1 (n = 26) | 1 ≤ Age < 6 (n = 50) | 6 ≤ Age < 12 (n = 82) | 12 ≤ Age < 18 (n = 97) | |
|---|---|---|---|---|---|
| Age (years), (Mean ± SD) | 9.05 ± 5.39 | 0.48 ± 0.27 | 3.06 ± 1.49 | 8.84 ± 1.81 | 14.61 ± 1.72 |
| Sex (boy), n (%) | 140(54.9) | 14 (53.8) | 28 (56.0) | 42 (51.2) | 56 (57.7) |
| Fever (≥38 °C) detection | |||||
| IRTT, n (%) | 40 (15.7) | 4 (15.3) | 13 (26.0) | 11 (13.4) | 12 (12.4) |
| NCFIT, n (%) | 44 (17.3) | 6 (23.1) | 9 (34.0) | 10 (12.2) | 11 (11.3) |
| Categories | NCFIT (a) | IRTT (b) | Difference (a–b) | t(p) | ICC (95%CI) | Kappa (95%CI) |
| Overall (n = 224) | 37.24 ± 0.62 | 37.39 ± 0.66 | −0.14 ± 0.29 | −7.99(<0.001) | 0.87 (0.75–0.95) | 0.82 (0.73–092) |
| age < 1 (n = 26) | 37.22 ± 0.70 | 37.44 ± 0.87 | −0.21 ± 0.34 | −3.28(0.003) | 0.87 (0.60–1.00) | 0.75 (0.438–1.00) |
| 1 ≤ age < 6 (n = 50) | 37.36 ± 0.71 | 37.57 ± 0.75 | −0.20 ± 0.26 | −5.41(<0.001) | 0.89 (0.70–1.00) | 0.81 (0.63–0.98) |
| 6 ≤ age < 12 (n = 82) | 37.17 ± 0.55 | 37.36 ± 0.49 | −0.19 ± 0.27 | −6.35(<0.001) | 0.81 (0.65–0.96) | 0.83 (0.65~1.00) |
| 12 ≤ age < 18 (n = 97) | 37.24 ± 0.62 | 37.30 ± 0.66 | −0.06 ± 0.30 | −2.07(0.042) | 0.88 (0.74–1.00) | 0.85 (0.68~1.00) |
| IRTT | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| ≥38 °C | <38 °C | |||||||
| Overall, (n = 255) | ||||||||
| NCFIT | ≥38 °C | 36 | 4 | 0.81 (0.70–0.93) | 0.98 (0.96–0.99) | 0.90 (0.80–0.99) | 0.96 (0.93–0.98) | 0.95 (0.92–0.97) |
| <38 °C | 8 | 207 | ||||||
| age < 1, (n = 26) | ||||||||
| NCFIT | ≥38 °C | 4 | 0 | 0.66 (0.28–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.90 (0.78–1.00) | 0.92 (0.82–1.00) |
| <38 °C | 2 | 20 | ||||||
| 1 ≤ age < 6, (n = 50) | ||||||||
| NCFIT | ≥38 °C | 13 | 0 | 0.76 (0.56–0.96) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.89 (0.79–0.99) | 0.92 (0.84–0.99) |
| <38 °C | 4 | 33 | ||||||
| 6 ≤ age < 12, (n = 82) | ||||||||
| NCFIT | ≥38 °C | 9 | 2 | 0.90 (0.71–1.00) | 0.97 (0.93–1.00) | 0.81 (0.59–1.00) | 0.98 (0.95–1.00) | 0.96 (0.92–1.00) |
| <38 °C | 1 | 70 | ||||||
| 12 ≤ age < 18, (n = 97) | ||||||||
| NCFIT | ≥38 °C | 10 | 2 | 0.90 (0.73–1.00) | 0.97 (0.94–1.00) | 0.83 (0.62–1.00) | 0.98 (0.96–1.00) | 0.96 (0.93–1.00) |
| <38 °C | 1 | 84 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Jang, M.-R.; Moon, J.-R.; Park, G.; An, Y.-J.; Seo, J.-M. Clinical Accuracy of Non-Contact Forehead Infrared Thermometer Measurement in Children: An Observational Study. Children 2022, 9, 1389. https://doi.org/10.3390/children9091389
Kim Y-M, Jang M-R, Moon J-R, Park G, An Y-J, Seo J-M. Clinical Accuracy of Non-Contact Forehead Infrared Thermometer Measurement in Children: An Observational Study. Children. 2022; 9(9):1389. https://doi.org/10.3390/children9091389
Chicago/Turabian StyleKim, Yeon-Mi, Myung-Roul Jang, Ju-Ryoung Moon, Goeun Park, Ye-Jin An, and Jeong-Meen Seo. 2022. "Clinical Accuracy of Non-Contact Forehead Infrared Thermometer Measurement in Children: An Observational Study" Children 9, no. 9: 1389. https://doi.org/10.3390/children9091389
APA StyleKim, Y.-M., Jang, M.-R., Moon, J.-R., Park, G., An, Y.-J., & Seo, J.-M. (2022). Clinical Accuracy of Non-Contact Forehead Infrared Thermometer Measurement in Children: An Observational Study. Children, 9(9), 1389. https://doi.org/10.3390/children9091389






