Intranasal Dexmedetomidine as Sedative for Medical Imaging in Young Children: A Systematic Review to Provide a Roadmap for an Evidence-Guided Clinical Protocol
Abstract
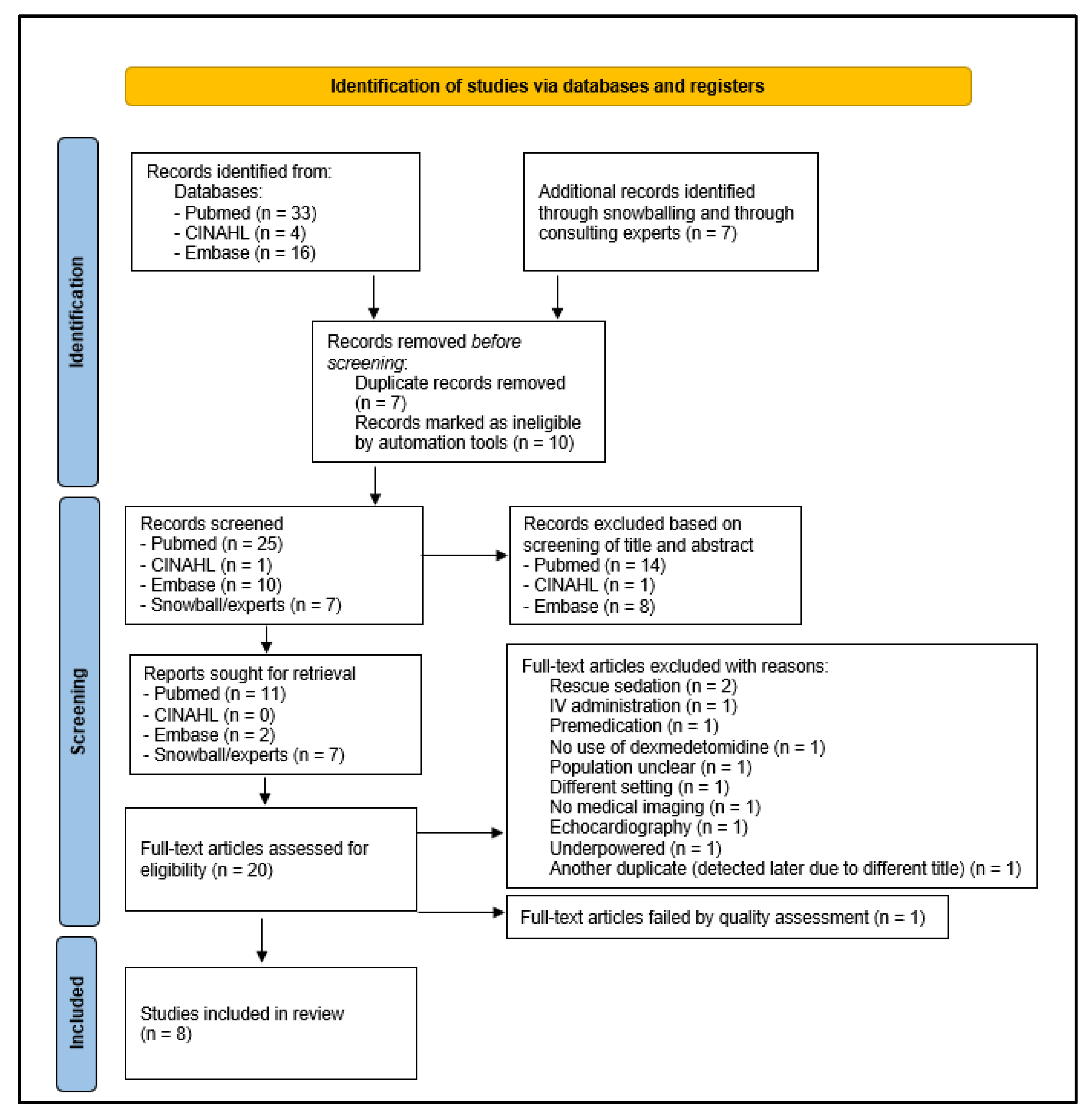
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria and Systematic Search Strategy
- Child, up to the age of five years;
- Procedural sedation;
- Dexmedetomidine;
- Intranasal administration; and
- Medical imaging.
2.2. Screening Process
2.3. Quality Assessment and Data Extraction
3. Results
3.1. Selection Criteria and Systematic Search Strategy
3.2. Quality Assessment
3.3. Data Extraction
3.3.1. Purpose of Studies and Outcomes
3.3.2. Study Design and Characteristics, Sample Size, and Inclusion and Exclusion Criteria
3.3.3. Method of Administration, Timing and Dose
3.3.4. Outcome Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. United Nations Human Rights. Available online: https://www.ohchr.org/en/instruments-mechanisms/instruments/convention-rights-child (accessed on 11 August 2022).
- Krauss, B.; Green, S.M. Procedural sedation and analgesia in children. Lancet 2006, 367, 766–780. [Google Scholar] [PubMed]
- Coté, C.J.; Wilson, S. Guidelines for Monitoring and Management of Pediatric Patients before, during, and after Sedation for Diagnostic and Therapeutic Procedures. Pediatrics 2019, 143, e20191000. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, L.; Allegaert, K.; Toelen, J.; Vanhonsebrouck, K. Pediatric Procedural Sedation and Analgesia (PROSA) in the Leuven university Hospitals: An audit on efficacy and safety. Children 2022, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Sims, M.J.; Robinson, L.C.; Titus, M.O.; Jackson, B.F. Pediatric Emergency Medicine Training in Procedural Sedation: Is It Time for a Standardized Curriculum? Pediatr. Emerg. Care 2021, 37, e1578–e1581. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.M.R.F.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Annex 1, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/dexdor-epar-product-information_en.pdf (accessed on 11 August 2022).
- Mason, K.P.; Lerman, J. Dexmedetomidine in Children. Anesth. Analg. 2011, 113, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, R.; Riggins, J.; Kariyanna, S.; Calkins, P.; Rotta, A.T. High-dose dexmedetomidine sedation for pediatric MRI. Pediatr. Anesth. 2011, 21, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sulton, C.; McCracken, C.; Simon, H.K.; Hebbar, K.; Reynolds, J.; Cravero, J.; Mallory, M.; Kamat, P. Pediatric Procedural Sedation Using Dexmedetomidine: A Report from the Pediatric Sedation Research Consortium. Hosp. Pediatr. 2016, 6, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Schrier, L.; Hadjipanayis, A.; Stiris, T.; Ross-Russell, R.I.; Valiulis, A.; Turner, M.A.; Zhao, W.; De Cock, P.; de Wildt, S.N.; Allegaert, K.; et al. Off-label use of medicines in neonates, infants, children, and adolescents: A joint policy statement by the European Academy of Paediatrics and the European Society for Developmental Perinatal and Pediatric Pharmacology. Eur. J. Pediatr. 2020, 179, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp (accessed on 11 August 2022).
- National Institute of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 11 August 2022).
- AMSTAR. Assessing the Methodological Quality of Systematic Reviews, the Development of AMSTAR. Available online: https://amstar.ca/index.php (accessed on 11 August 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ghai, B.; Jain, K.; Saxena, A.K.; Bhatai, N.; Sodhi, K.S. Comparison of oral midazolam with intranasal dexmedetomidine premedication for children undergoing CT imaging: A randomized, double-blind, and controlled study. Pediatr. Anesth. 2016, 27, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tug, A.; Hanci, A.; Turk, H.S.; Aybey, F.; Isil, C.T.; Sayin, P.; Oba, S. Comparison of Two Different Intranasal Doses of Dexmedetomidine in Children for Magnetic Resonance Imaging Sedation. Paediatr. Drugs 2015, 17, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.M.; Li, B.L.; Cheuk, D.K.; Leung, M.K.M.; Hui, T.W.C.; Wong, I.C.; Lam, W.W.; Choi, S.W.; Irwin, M.G. A randomised controlled trial of oral chloral hydrate vs. intranasal dexmedetomidine before computerised tomography in children. Anaesthesia 2017, 72, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.J.; Dawes, D.; Ahmad, S.; Martin, D.; Gyamtso, C. Dexmedetomidine improves success of paediatric MRI sedation. Arch. Dis. Child. 2022, 107, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Ambi, U.; Joshi, C.; Ganeshnavar, A.; Adrash, E. Intranasal dexmedetomidine for paediatric sedation for diagnostic magnetic resonance imaging studies. Indian J. Anaesth. 2012, 56, 587. [Google Scholar] [CrossRef] [PubMed]
- Filho, E.M.; Robinson, F.; De Carvalho, W.B.; Gilio, A.E.; Mason, K.P. Intranasal Dexmedetomidine for Sedation for Pediatric Computed Tomography Imaging. J. Pediatr. 2015, 166, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- Uusalo, P.; Guillaume, S.; Siren, S.; Manner, T.; Vilo, S.; Scheinin, M.; Saari, T.I. Pharmacokinetics and Sedative Effects of Intranasal Dexmedetomidine in Ambulatory Pediatric Patients. Anesth. Analg. 2020, 130, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Sulton, C.; Kamat, P.; Mallory, M.; Reynolds, J. The Use of Intranasal Dexmedetomidine and Midazolam for Sedated Magnetic Resonance Imaging in Children: A report from the pediatric sedation research consortium. Pediatr. Emerg. Care 2020, 36, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Bailey, C.R. Intranasal dexmedetomidine for sedation in children: A review. J. Perioper. Pract. 2020, 30, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.P. Sedation for Radiological Procedures. In Pediatric Sedation Outside of the Operating Room. A Multispecialty International Collaboration, 3rd ed.; Mason, K.P., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 475–496. [Google Scholar]
| Aspect | Suggestions | |
|---|---|---|
| Contraindications | ASA-status III/IV; hepatic abnormalities; cardiac abnormalities; central nervous system dysfunction; respiratory or renal dysfunction; risk of difficult intubation; drug allergies | |
| Dose | Age-related suggestion: 2,5 µg/kg under 1 year, 3 µg/kg in 1 to 3 years, 4 µg/kg in 3 to 5 years Modality-related suggestion: CT: 2.5–3 µg/kg; MRI 2–4 µg/kg | |
| Administration method | Nebulization by mucosal spray | |
| Timing of administration | 30–45 min before the procedure | |
| NPO policy | Variability in practices
| |
| Sedation monitoring | A valid sedation scale should be used. Consider the use of the Ramsay sedation score (RSS) or the University of Michigan Sedation Scale 10 and 20 min after administration, respectively. For the RSS score and to obtain adequate sedation, this score should be as follows: for MRI: ≥4 or ≥5; for CT: ≥3 or ≥4. | |
| Rescue medication | Several options were suggested in the reviewed studies, including (1) an additional dose of dexmedetomidine, (2) intravenous bolus propofol or thiopental or (3) intravenous/intranasal midazolam. Owing to this inconsistency, we cannot provide further guidance on selection of rescue medication. | |
| Discharge | Modified Aldrete score has to be registered after the procedure (threshold score ≥9) for discharge. | |
| Monitoring | Continuous monitoring of heart rate and oxygen saturation is highly recommended throughout the procedure. If an abnormal heart rate is observed, blood pressure should also be measured. Blood pressure is not measured by default, as it can result in arousal and is a late indicator of circulatory failure. | |
| Potential side effects | Commonly occurring | Bradycardia, with a decrease of <20% |
| Hypotension, with a decrease of <20% | ||
| Less commonly occurring | Bradycardia, with a decrease of >20% | |
| Hypotension, with a decrease of >20% | ||
| Vomiting | ||
| Rarely occurring | Desaturation, hypertension and tachycardia | |
| Non-pharmacological interventions | Consider simultaneous use of non-pharmacological interventions, such as hearing protection, use of a vacuum matress or a distraction or mockup | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermans, K.; Ramaekers, L.; Toelen, J.; Vanhonsebrouck, K.; Allegaert, K. Intranasal Dexmedetomidine as Sedative for Medical Imaging in Young Children: A Systematic Review to Provide a Roadmap for an Evidence-Guided Clinical Protocol. Children 2022, 9, 1310. https://doi.org/10.3390/children9091310
Hermans K, Ramaekers L, Toelen J, Vanhonsebrouck K, Allegaert K. Intranasal Dexmedetomidine as Sedative for Medical Imaging in Young Children: A Systematic Review to Provide a Roadmap for an Evidence-Guided Clinical Protocol. Children. 2022; 9(9):1310. https://doi.org/10.3390/children9091310
Chicago/Turabian StyleHermans, Kato, Larissa Ramaekers, Jaan Toelen, Koen Vanhonsebrouck, and Karel Allegaert. 2022. "Intranasal Dexmedetomidine as Sedative for Medical Imaging in Young Children: A Systematic Review to Provide a Roadmap for an Evidence-Guided Clinical Protocol" Children 9, no. 9: 1310. https://doi.org/10.3390/children9091310
APA StyleHermans, K., Ramaekers, L., Toelen, J., Vanhonsebrouck, K., & Allegaert, K. (2022). Intranasal Dexmedetomidine as Sedative for Medical Imaging in Young Children: A Systematic Review to Provide a Roadmap for an Evidence-Guided Clinical Protocol. Children, 9(9), 1310. https://doi.org/10.3390/children9091310







