Safety Evaluation of Oral Sirolimus in the Treatment of Childhood Diseases: A Systematic Review
Abstract
:1. Introduction
2. Method
2.1. Search Strategy
2.2. Inclusion Criteria and Exclusion Criteria
2.3. Data Extraction
2.4. Differences between the Protocol and the Work That Was Performed
2.5. Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
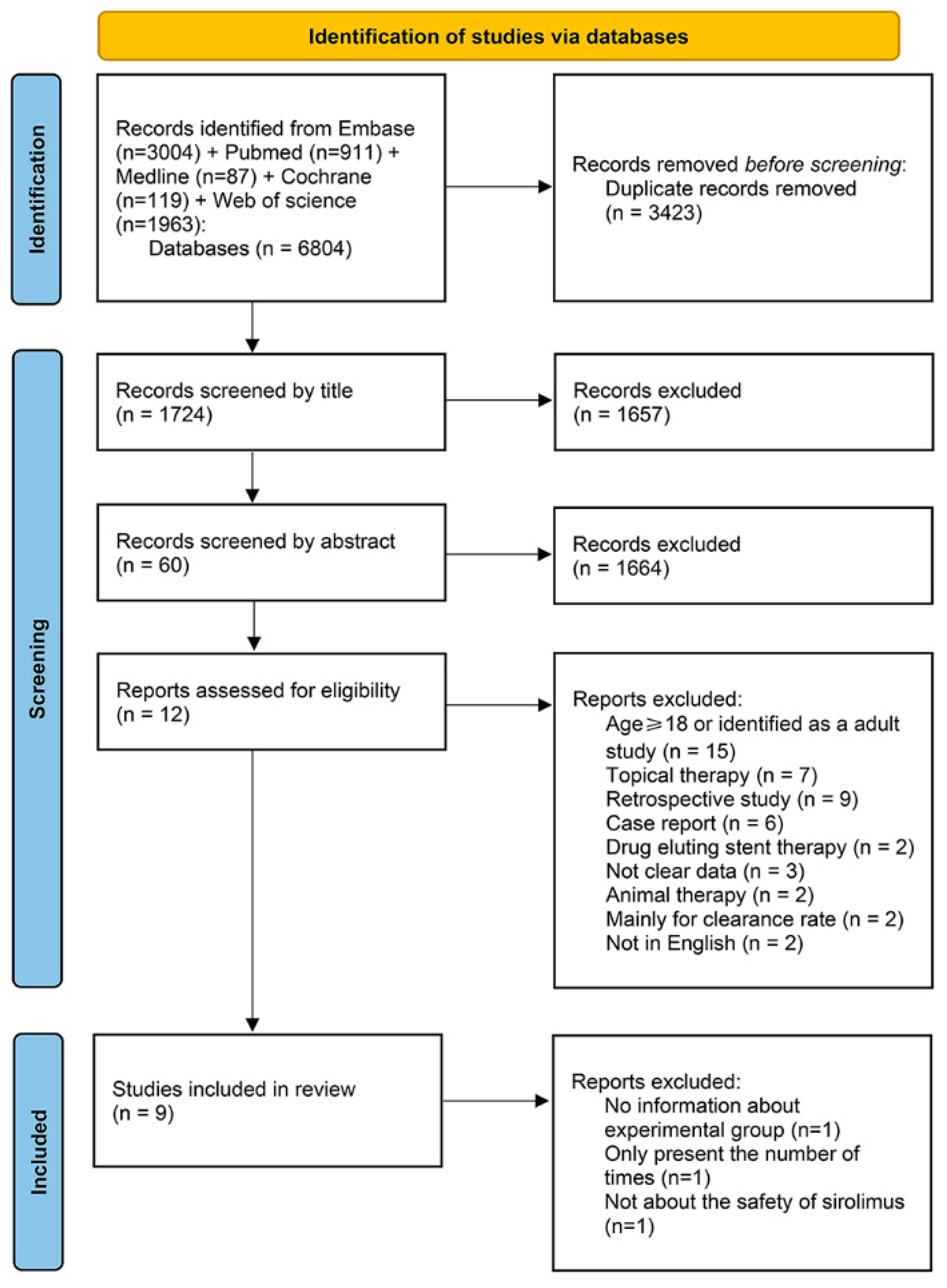
3.1. Search Results and Characteristics of the Included Studies
3.2. The Risk of Bias
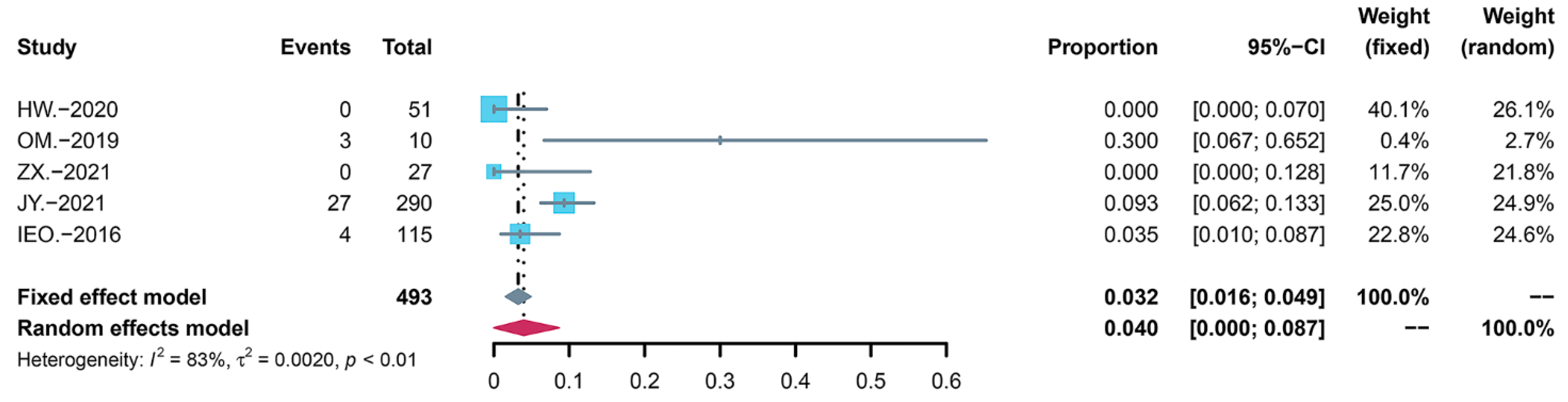
3.3. Summary of the Incidence of AEs
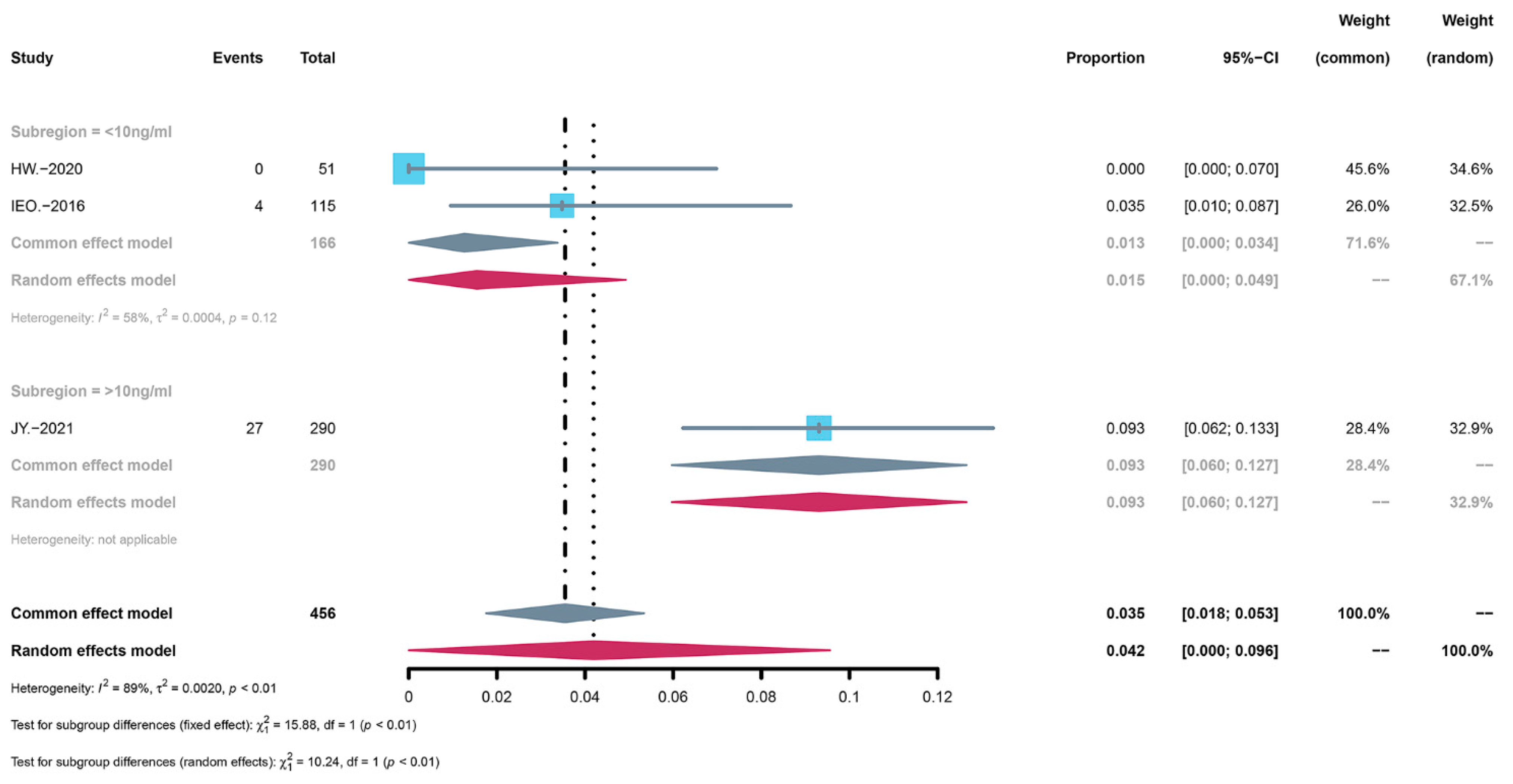
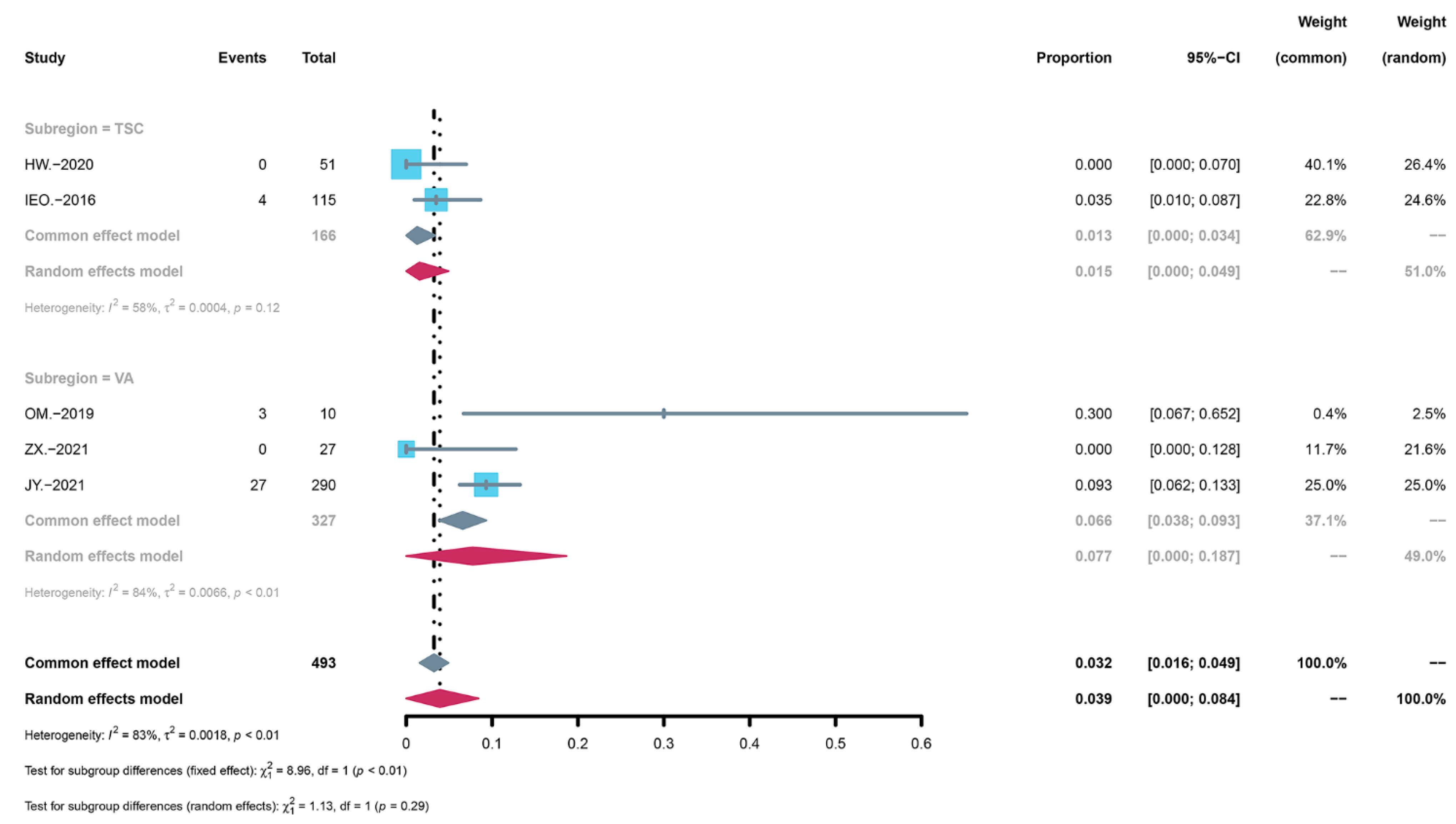
3.4. Subgroup Analysis of AEs
3.5. Summary of the Severity of AEs
3.6. Subgroup Analysis of the Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vezina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 1975, 28, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N.; Baker, H.; Vezina, C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiot. 1975, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xie, W.; Zhang, Z. Efficacy and safety of sirolimus in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020, 50, 1073–1080. [Google Scholar] [CrossRef]
- Kahan, B.D. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: A randomised multicentre study. The Rapamune US Study Group. Lancet 2000, 356, 194–202. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef]
- Granata, S.; Dalla Gassa, A.; Carraro, A.; Brunelli, M.; Stallone, G.; Lupo, A.; Zaza, G. Sirolimus and Everolimus Pathway: Reviewing Candidate Genes Influencing Their Intracellular Effects. Int. J. Mol. Sci. 2016, 17, 735. [Google Scholar] [CrossRef]
- Sehgal, S.N. Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998, 31, 335–340. [Google Scholar] [CrossRef]
- Sandbank, S.; Molho-Pessach, V.; Farkas, A.; Barzilai, A.; Greenberger, S. Oral and Topical Sirolimus for Vascular Anomalies: A Multicentre Study and Review. Acta Derm. Venereol. 2019, 99, 990–996. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.; Richard, D.; Laplante, M. The Roles of mTOR Complexes in Lipid Metabolism. Annu. Rev. Nutr. 2015, 35, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Prudent, J.; Basu, K.; Goyon, V.; Katsumura, S.; Hulea, L.; Pearl, D.; Siddiqui, N.; Strack, S.; McGuirk, S.; et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol. Cell 2017, 67, 922–935.e925. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Han, D.; Wang, D.; Lu, H.; Wang, X. Efficacy and safety of sirolimus in the treatment of vascular malformations: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e22596. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Yang, K.; Xia, C.; Li, L. Kaposiform hemangioendothelioma: Current knowledge and future perspectives. Orphanet J. Rare Dis. 2020, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.M.; Trenor, C.C., 3rd; Hammill, A.M.; Vinks, A.A.; Patel, M.N.; Chaudry, G.; Wentzel, M.S.; Mobberley-Schuman, P.S.; Campbell, L.M.; Brookbank, C.; et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016, 137, e20153257. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, S.; Théorêt, Y.; Dubois, J.; Essouri, S.; Pincivy, A.; Coulombe, J.; McCuaig, C.; Powell, J.; Soulez, G.; Kleiber, N. Systemic, local, and sclerotherapy drugs: What do we know about drug prescribing in vascular anomalies? Pediatric Blood Cancer 2021, 68, e29364. [Google Scholar] [CrossRef]
- Freixo, C.; Ferreira, V.; Martins, J.; Almeida, R.; Caldeira, D.; Rosa, M.; Costa, J.; Ferreira, J. Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic review. J. Vasc. Surg. 2020, 71, 318–327. [Google Scholar] [CrossRef]
- Zhang, A.; Duchatelet, S.; Lakdawala, N.; Tower, R.L.; Diamond, C.; Marathe, K.; Hill, I.; Richard, G.; Diab, Y.; Kirkorian, A.Y.; et al. Targeted Inhibition of the Epidermal Growth Factor Receptor and Mammalian Target of Rapamycin Signaling Pathways in Olmsted Syndrome. JAMA Dermatol. 2020, 156, 196–200. [Google Scholar] [CrossRef]
- Nadal, M.; Giraudeau, B.; Tavernier, E.; Jonville-Bera, A.P.; Lorette, G.; Maruani, A. Efficacy and Safety of Mammalian Target of Rapamycin Inhibitors in Vascular Anomalies: A Systematic Review. Acta Derm. Venereol. 2016, 96, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Kleiber, N.; Gariépy-Assal, L.; Coulombe, J.; Marcoux, S.; Essouri, S.; McCuaig, C.; Powell, J.; Soulez, G.; Dubois, J. Off-Label Use and Safety of Drug Use in Vascular Anomalies. Dermatology 2021, 237, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, S.; Yang, K.; Zhou, J.; Zhang, X.; Jiang, X.; Xu, X.; Lu, G.; Qiu, L.; Kong, F.; et al. A prospective multicenter study of sirolimus for complicated vascular anomalies. J. Vasc. 2021, 74, 1673–1681.e1673. [Google Scholar] [CrossRef] [PubMed]
- Cardamone, M.; Flanagan, D.; Mowat, D.; Kennedy, S.E.; Chopra, M.; Lawson, J.A. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J. Pediatr. 2014, 164, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Liern, M.; De Reyes, V.; Fayad, A.; Vallejo, G. Use of sirolimus in patients with primary steroid-resistant nephrotic syndrome. Nefrologia 2012, 32, 321–328. [Google Scholar] [CrossRef]
- Maruani, A.; Tavernier, E.; Boccara, O.; Mazereeuw-Hautier, J.; Leducq, S.; Bessis, D.; Guibaud, L.; Vabres, P.; Carmignac, V.; Mallet, S.; et al. Sirolimus (Rapamycin) for Slow-Flow Malformations in Children: The Observational-Phase Randomized Clinical PERFORMUS Trial. JAMA Dermatol. 2021, 157, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.S.; Hisham, A.R.B.; Ch Er, C.I.Y.; Cheah, K.K.; Ghani, N.; Noorani, T.Y. Success rates of coronal and partial pulpotomies in mature permanent molars: A systematic review and single-arm meta-analysis. Quintessence Int. 2021, 52, 196–208. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Nunes, R.G.; Santiago-Junior, J.F.; Marcela de Luna Gomes, J.; Oliveira Limirio, J.P.J.; Rosa, C.; Verri, F.R.; Pellizzer, E.P. Are implant-supported removable partial dentures a suitable treatment for partially edentulous patients? A systematic review and meta-analysis. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wang, Y.Y.; Zhang, M.N.; Lu, Q.; Pang, L.Y.; Liu, L.Y.; Li, Y.F.; Zou, L.P. Sirolimus Can Increase the Disappearance Rate of Cardiac Rhabdomyomas Associated with Tuberous Sclerosis: A Prospective Cohort and Self-Controlled Case Series Study. J. Pediatr. 2021, 233, 150–155.e154. [Google Scholar] [CrossRef]
- He, W.; Chen, J.; Wang, Y.Y.; Zhang, M.N.; Qian, L.; Wang, Q.H.; Luo, X.M.; Chen, X.Q.; Zou, L.P. Sirolimus improves seizure control in pediatric patients with tuberous sclerosis: A prospective cohort study. Seizure 2020, 79, 20–26. [Google Scholar] [CrossRef]
- Ozeki, M.; Nozawa, A.; Yasue, S.; Endo, S.; Asada, R.; Hashimoto, H.; Fukao, T. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J. Rare Dis. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Guo, Y.; Liu, Y.; Zhang, J.; Li, Y.; Liu, Q.; Liu, Z.; Sun, N.; Li, X.; et al. Efficacy of Initial Sirolimus Therapy for 27 Patients with Intractable Lymphatic Malformations. Laryngoscope 2021, 131, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Overwater, I.E.; Rietman, A.B.; Bindels-de Heus, K.; Looman, C.W.; Rizopoulos, D.; Sibindi, T.M.; Cherian, P.J.; Jansen, F.E.; Moll, H.A.; Elgersma, Y.; et al. Sirolimus for epilepsy in children with tuberous sclerosis complex: A randomized controlled trial. Neurology 2016, 87, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Blydt-Hansen, T.D.; Gibson, I.W.; Birk, P.E. Histological progression of chronic renal allograft injury comparing sirolimus and mycophenolate mofetil-based protocols. A single-center, prospective, randomized, controlled study. Pediatr. Transplant. 2010, 14, 909–918. [Google Scholar] [CrossRef]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.; Cantor, A.; Perentesis, J.; Schorry, E.; Ullrich, N.; Gutmann, D.H.; et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: A neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015, 17, 596–603. [Google Scholar] [CrossRef]
- Tejani, A.; Alexander, S.; Ettenger, R.; Lerner, G.; Zimmerman, J.; Kohaut, E.; Briscoe, D.M. Safety and pharmacokinetics of ascending single doses of sirolimus (Rapamune, rapamycin) in pediatric patients with stable chronic renal failure undergoing dialysis. Pediatr. Transplant. 2004, 8, 151–160. [Google Scholar] [CrossRef]
- Mariani, L.G.; Schmitt, I.R.; Garcia, C.D.; Kiszewski, A.E. Low dose sirolimus treatment for refractory tufted angioma and congenital kaposiform hemangioendothelioma, both with Kasabach-Merritt phenomenon. Pediatric Blood Cancer 2019, 66, e27810. [Google Scholar] [CrossRef]
- Laugharne, M.; Cross, S.; Richards, S.; Dawson, C.; Ilchyshyn, L.; Saleem, M.; Mathieson, P.; Smith, R. Sirolimus toxicity and vascular endothelial growth factor release from islet and renal cell lines. Transplantation 2007, 83, 1635–1638. [Google Scholar] [CrossRef]
- Kloster-Jensen, K.; Sahraoui, A.; Vethe, N.T.; Korsgren, O.; Bergan, S.; Foss, A.; Scholz, H. Treatment with Tacrolimus and Sirolimus Reveals No Additional Adverse Effects on Human Islets In Vitro Compared to Each Drug Alone but They Are Reduced by Adding Glucocorticoids. J. Diabetes Res. 2016, 2016, 4196460. [Google Scholar] [CrossRef]
- Morelon, E.; Stern, M.; Israel-Biet, D.; Correas, J.M.; Danel, C.; Mamzer-Bruneel, M.F.; Peraldi, M.N.; Kreis, H. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation 2001, 72, 787–790. [Google Scholar] [CrossRef]
- Avitzur, Y.; Jimenez-Rivera, C.; Fecteau, A.; Jones, N.; Ngan, B.Y.; Ng, V.L. Interstitial granulomatous pneumonitis associated with sirolimus in a child after liver transplantation. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Garrod, A.S.; Goyal, R.K.; Weiner, D.J. Sirolimus-induced interstitial lung disease following pediatric stem cell transplantation. Pediatr. Transplant. 2015, 19, E75–E77. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year of Publication | Country | The Source of Funding | Study Type | Patients (n) | Age | Sex, n | Diseases | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (Year) | Range (Year) | Male | Female | |||||||
| Cardamone, M [23] | 2014 | Australia | Novartis | Single-center open-label | 7 | 6 | 3–17 | 3 | 4 | Tuberous sclerosis complex |
| Chen, X. Q. [29] | 2021 | China | The National Key Research and Development Program of China (No. 2016YFC1000707) and The National Natural Science Foundation of China (No. 81471329) | Prospective cohort study | 217 | 6 | 2–23 | 121 | 96 | Tuberous sclerosis complex |
| He, W. [30] | 2020 | China | The National Key Research and Development Program of China (2016YFC1000707) | Prospective cohort study | 91 | 2 | 0–12 | 47 | 44 | Tuberous sclerosis complex |
| Ozeki, M. [31] | 2019 | Japan | A Clinical research-clinical trial promotion research project (18lk0201055h0003) and Practical Research Project for Rare/Intractable Diseases (18ek0109277h0002) | Prospective study | 12 | 6.5 | 0.04–18 | 6 | 6 | Lymphatic anomalies |
| Zhang, X. [32] | 2021 | China | Beijing Hospitals Authority’ Ascent Plan (DFL20191201) and Beijing Hospitals Authority Youth Program (QML20181202) | Prospective open-label study | 27 | 2.3 | 0–15 | 12 | 15 | Lymphatic anomalies |
| Liern, M. [24] | 2012 | Argentina | - | Prospective cohort study | 13 | 10 | 8–18 | 4 | 9 | Nephrotic syndrome |
| Ji, Y. [22] | 2021 | China | The National Natural Science Foundation of China (81400862 and 81401606), the Key Project in the Science & Technology Program of Sichuan Province (2019YFS0322), etc. | Multicenterphase II trial | 126 | 4.8 | 0–14 | 64 | 62 | Vascular anomalies |
| Iris E. Overwater [33] | 2016 | Netherlands | The Dutch Epilepsy Foundation | Randomized controlled study | 23 | 5.5 | 1.8–10.9 | 11 | 12 | Tuberous sclerosis complex |
| Marua, A. [25] | 2021 | France | The French Ministry of Social Affairs and Health (French National Program of Clinical Research [PHRC-N], 2014) | Randomized controlled study | 59 | 11.6y | 6–18y | 24 | 35 | Slow-flow vascular malformations |
| Summary | 575 | 292 | 283 | |||||||
| First Author | Intervention | Treatment Duration | |||
|---|---|---|---|---|---|
| Starting Dose | Regimen | Targeted Blood Concentration | Range | Median | |
| Cardamone, M [23] | 1 mg/m2 /d | — | 4–10 ng/ml | 6–36 months | 18 months |
| Chen, X. Q. [29] | 1 mg/m2 /d | — | 5–10 ng/ml | 7–22 months | 13 months |
| He, W. [30] | 1 mg/m2 /d | qd | 5–10 ng/ml | — | — |
| Ozeki, M. [31] | BSA ≥ 1.0 m2 2 mg/d BSA< 1.0 m2 1 mg/d | qd | 5–15 ng/ml | 6–30 months | 12.5 months |
| Zhang, X. [32] | 0.5 mg/m2 /d | qd | 4–13 ng/ml | 6–27 months | 10.6 months |
| Liern, M. [24] | 1 mg/m2 /d | qd | 7–10 ng/ml | 12 months | 12 months |
| Ji, Y. [22] | 0.8 mg/m2 | bid | 10–15 ng/ml | 0.4–4.5 years | 3 years |
| Iris E. Overwater [33] | — | — | 5–10 ng/ml | 6 months | 6 months |
| Marua, A. [25] | 0.08 mg/kg/d | bid | 4–12 ng/ml | 12 months | 12 months |
| Studies | Selection | Comparability | Outcome | Total Quality Score | Level b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Exposed Cohort | Nonexposed Cohort | Ascertainment of Exposure | Outcome of Interest Not Present at Star | Main Factor | Additional Factor | Assessment of Outcome | Follow-Up Long Enough | Adequacy of Follow-Up of Cohorts | ||
| Cardamone, M [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Chen, X. Q. [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| He, W. [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Ozeki, M. [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Zhang, X. [32] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Liern, M. [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Ji, Y. [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Iris E. Overwater [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Marua, A. [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | Good |
| Sirolimus (Total Patients n = 575) | ||
|---|---|---|
| n a | % | |
| Patients with at least 1 adverse event | ||
| Oral mucositis | 118 | 20.52 |
| Acne | 25 | 4.35 |
| Pneumonia | 26 | 4.52 |
| Upper respiratory tract infection | 94 | 16.35 |
| Lymph node infection | 5 | 0.87 |
| Otitis media | 2 | 0.35 |
| Other infection | 12 | 2.09 |
| Fever | 6 | 1.04 |
| Gastrointestinal reaction | 53 | 9.22 |
| ♦ Nausea and vomiting | 30 | 5.22 |
| ♦ Diarrhea | 13 | 2.26 |
| Anorexia | 6 | 1.04 |
| Cellulitis | 1 | 0.17 |
| Rash | 10 | 1.74 |
| Eczema | 17 | 2.96 |
| Pain | 35 | 6.09 |
| ♦ Headache | 17 | 2.96 |
| ♦ Muscle pain | 1 | 0.17 |
| Dizziness | 1 | 0.17 |
| Hypertension | 4 | 0.70 |
| Edema | 4 | 0.70 |
| Hemorrhagic disease | 4 | 0.70 |
| Fatigue | 4 | 0.70 |
| Alopecia | 5 | 0.87 |
| Hyperhidrosis | 3 | 0.52 |
| Polyuria | 1 | 0.17 |
| Wound healing delay | 4 | 0.70 |
| Red eye | 1 | 0.17 |
| Behavioral change | 3 | 0.52 |
| Injury due to accident | 4 | 0.70 |
| Laboratory | ||
| Dyslipidemia | 36 | 6.26 |
| ♦ Hypercholesterolemia | 17 | 2.96 |
| ♦ Hyperlipidemia | 23 | 4.00 |
| ♦ Elevated LDL | 7 | 1.22 |
| Anemia | 6 | 1.04 |
| Neutropenia | 15 | 2.61 |
| Lymphocytopenia | 8 | 1.39 |
| Thrombocytosis | 25 | 4.35 |
| Increases in liver enzymes | 53 | 9.22 |
| ♦ spartate aminotransferase raised | 3 | 0.52 |
| ♦ Alanine aminotransferase raised | 2 | 0.35 |
| Adverse Events a | Incidence Rate | |||
|---|---|---|---|---|
| Before Deletion | After Deletion b | |||
| I2 | Incidence Rate c | I2 | Incidence Rate c | |
| Gastrointestinal reaction | 95% | 14.5% (95%CI: 0.044–0.245) | 25% | 0.1% (95%CI: 0.000–0.007) |
| ♦ Nausea and vomiting | 89% | 4.8% (95%CI: 0.000–0.110) | 0% | 0.1% (95%CI: 0.000–0.007) |
| ♦ Diarrhea | 71% | 1.4% (95%CI: 0.000–0.036) | 0% | 0.0% (95%CI: 0.000–0.006) |
| Oral mucositis | 91% | 21.9% (95%CI: 0.112–0.325) | 32% | 8.2% (95%CI: 0.054–0.110) |
| Acne | 91% | 3.8% (95%CI: 0.000–0.076) | 26% | 0.1% (95%CI: 0.000–0.007) |
| Upper respiratory tract infection | 96% | 21.2% (95%CI: 0.076–0.347) | 51% | 3.5% (95%CI: 0.000–0.082) |
| Pneumonia | 78% | 2.2% (95%CI: 0.000–0.050) | 0% | 0.0% (95%CI: 0.000–0.006) |
| Anorexia | 0% | 0.1% (95%CI: 0.000–0.006) | 0% | 0.1% (95%CI: 0.000–0.006) |
| Fatigue | 0% | 0.0% (95%CI: 0.000–0.005) | 0% | 0.0% (95%CI: 0.000–0.005) |
| Pain | 84% | 3.3% (95%CI: 0.003–0.064) | 71% | 1.5% (95%CI: 0.000–0.038) |
| ♦ Headache | 65% | 0.6% (95%CI: 0.000–0.021) | 0% | 0.0% (95%CI: 0.000–0.005) |
| Edema | 0% | 0.1% (95%CI: 0.000–0.007) | 0% | 0.1% (95%CI: 0.000–0.007) |
| Alopecia | 0% | 0.1% (95%CI: 0.000–0.007) | 0% | 0.1% (95%CI: 0.000–0.007) |
| Eczema | 62% | 1.4% (95%CI: 0.000–0.035) | 0% | 0.0% (95%CI: 0.000–0.006) |
| Dyslipidemia | 0% | 4.7% (95%CI: 0.027–0.067) | 0% | 4.7% (95%CI: 0.027–0.067) |
| ♦ Hypercholesterolemia | 61% | 1.4% (95%CI: 0.000–0.031) | 50% | 0.1% (95%CI: 0.000–0.007) |
| ♦ Hyperlipidemia | 0% | 3.9% (95%CI: 0.021–0.057) | 0% | 3.9% (95%CI: 0.021–0.057) |
| Anemia | 0% | 0.2% (95%CI: 0.000–0.008) | 0% | 0.2% (95%CI: 0.000–0.008) |
| Neutropenia | 54% | 1.9% (95%CI: 0.000–0.045) | 0% | 0.1% (95%CI: 0.000–0.007) |
| Increases in liver enzymes | 71% | 7.3% (95%CI: 0.022–0.125) | 35% | 5.3% (95%CI: 0.029–0.078) |
| First Author | Patients (n) | Grades (n) | ||
|---|---|---|---|---|
| All | Grade Ⅰ–Ⅱ | Grade Ⅲ–Ⅳ | ||
| He, W. | 91 | 51 | 51 | 0 |
| Ozeki, M. | 12 | 10 | 7 | 3 |
| Zhang, X. | 27 | 27 | 27 | 0 |
| Ji, Y. | 126 | 290 | 263 | 27 |
| Iris E. Overwater | 23 | 115 | 111 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Y.; Zhang, G.; Yang, K.; Qiu, T.; Zhou, J.; Gong, X.; Ji, Y. Safety Evaluation of Oral Sirolimus in the Treatment of Childhood Diseases: A Systematic Review. Children 2022, 9, 1295. https://doi.org/10.3390/children9091295
Zhang Z, Li Y, Zhang G, Yang K, Qiu T, Zhou J, Gong X, Ji Y. Safety Evaluation of Oral Sirolimus in the Treatment of Childhood Diseases: A Systematic Review. Children. 2022; 9(9):1295. https://doi.org/10.3390/children9091295
Chicago/Turabian StyleZhang, Zixin, Yanan Li, Guangyue Zhang, Kaiying Yang, Tong Qiu, Jiangyuan Zhou, Xue Gong, and Yi Ji. 2022. "Safety Evaluation of Oral Sirolimus in the Treatment of Childhood Diseases: A Systematic Review" Children 9, no. 9: 1295. https://doi.org/10.3390/children9091295
APA StyleZhang, Z., Li, Y., Zhang, G., Yang, K., Qiu, T., Zhou, J., Gong, X., & Ji, Y. (2022). Safety Evaluation of Oral Sirolimus in the Treatment of Childhood Diseases: A Systematic Review. Children, 9(9), 1295. https://doi.org/10.3390/children9091295







