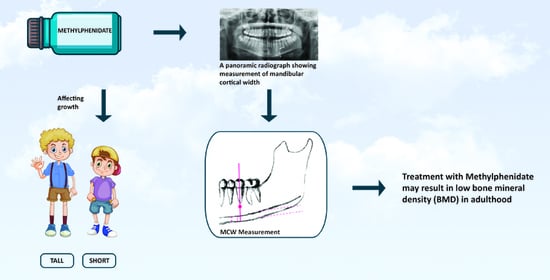
Possible Association between Methylphenidate and Mandibular Bone Characteristics Detected by Dental Panoramic Radiograph in Children and Adolescents with ADHD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. MCW Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef] [PubMed]
- Nøvik, T.S.; Hervas, A.; Ralston, S.J.; Dalsgaard, S.; Rodrigues Pereira, R.; Lorenzo, M.J. Influence of gender on attention-deficit/hyperactivity disorder in Europe—ADORE. Eur. Child Adolesc. Psychiatry 2006, 15 (Suppl. 1), I15–I24. [Google Scholar] [CrossRef] [PubMed]
- Ramtekkar, U.P.; Reiersen, A.M.; Todorov, A.A.; Todd, R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 217–228.e3. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E. Sex differences in ADHD: Conference summary. J. Abnorm. Child Psychol. 1996, 24, 555–569. [Google Scholar] [CrossRef]
- Gaub, M.; Carlson, C.L. Gender differences in ADHD: A meta-analysis and critical review. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 1036–1045. [Google Scholar] [CrossRef]
- Fraticelli, S.; Caratelli, G.; De Berardis, D.; Ducci, G.; Pettorruso, M.; Martinotti, G.; Di Cesare, G.; di Giannantonio, M. Gender differences in attention deficit hyperactivity disorder: An update of the current evidence. Riv. Psichiatr. 2022, 57, 159–164. [Google Scholar] [CrossRef]
- Mowlem, F.D.; Rosenqvist, M.A.; Martin, J.; Lichtenstein, P.; Asherson, P.; Larsson, H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 2019, 28, 481–489. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Wolraich, M.L.; Hagan, J.F., Jr.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef]
- Spencer, R.C.; Devilbiss, D.M.; Berridge, C.W. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol. Psychiatry 2015, 77, 940–950. [Google Scholar] [CrossRef] [Green Version]
- Schachar, R.J.; Tannock, R.; Cunningham, C.; Corkum, P.V. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Storebo, O.J.; Pedersen, N.; Ramstad, E.; Kielsholm, M.L.; Nielsen, S.S.; Krogh, H.B.; Moreira-Maia, C.R.; Magnusson, F.L.; Holmskov, M.; Gerner, T.; et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents—Assessment of adverse events in non-randomised studies. Cochrane Database Syst. Rev. 2018, 5, CD012069. [Google Scholar] [CrossRef] [PubMed]
- Cevikaslan, A.; Parlak, M.; Ellidag, H.Y.; Kulaksizoglu, S.C.; Yilmaz, N. Effects of methylphenidate on height, weight and blood biochemistry parameters in prepubertal boys with attention deficit hyperactivity disorder: An open label prospective study. Scand. J. Child. Adolesc. Psychiatr. Psychol. 2021, 9, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, F.; Gurbuz, B.B.; Celik, G.G.; Yildirim, V.; Ucakturk, S.A.; Seydaoglu, G.; Ucakturk, E.M.; Topaloglu, A.K.; Yuksel, B. Effects of methylphenidate on appetite and growth in children diagnosed with attention deficit and hyperactivity disorder. J. Pediatric Endocrinol. Metab. 2016, 29, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Koonrungsesomboon, K.; Koonrungsesomboon, N. The Effects of Methylphenidate Treatment on Child Growth in Thai Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2020, 30, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bereket, A.; Turan, S.; Karaman, M.G.; Haklar, G.; Ozbay, F.; Yazgan, M.Y. Height, weight, IGF-I, IGFBP-3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: Effect of methylphenidate treatment. Horm. Res. 2005, 63, 159–164. [Google Scholar] [CrossRef]
- Dura-Trave, T.; Yoldi-Petri, M.E.; Gallinas-Victoriano, F.; Zardoya-Santos, P. Effects of osmotic-release methylphenidate on height and weight in children with attention-deficit hyperactivity disorder (ADHD) following up to four years of treatment. J. Child Neurol. 2012, 27, 604–609. [Google Scholar] [CrossRef]
- Kang, K.D.; Yun, S.W.; Chung, U.; Kim, T.H.; Park, J.H.; Park, I.H.; Han, D.H. Effects of methylphenidate on body index and physical fitness in Korean children with attention deficit hyperactivity disorder. Hum. Psychopharmacol. 2016, 31, 76–82. [Google Scholar] [CrossRef]
- Zeiner, P. Body growth and cardiovascular function after extended treatment (1.75 years) with methylphenidate in boys with attention-deficit hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 1995, 5, 129–138. [Google Scholar] [CrossRef]
- Zhang, H.; Du, M.; Zhuang, S. Impact of long-term treatment of methylphenidate on height and weight of school age children with ADHD. Neuropediatrics 2010, 41, 55–59. [Google Scholar] [CrossRef]
- Satterfield, J.H.; Cantwell, D.P.; Schell, A.; Blaschke, T. Growth of hyperactive children treated with methylphenidate. Arch. Gen. Psychiatry 1979, 36, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Charach, A.; Figueroa, M.; Chen, S.; Ickowicz, A.; Schachar, R. Stimulant treatment over 5 years: Effects on growth. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Akay, A. The effects of methylphenidate on weight, height, and body mass index in Turkish children and adolescents with ADHD. Alpha Psychiatry 2020, 21, 211–217. [Google Scholar] [CrossRef]
- Diez-Suarez, A.; Vallejo-Valdivielso, M.; Marin-Mendez, J.J.; de Castro-Manglano, P.; Soutullo, C.A. Weight, Height, and Body Mass Index in Patients with Attention-Deficit/Hyperactivity Disorder Treated with Methylphenidate. J. Child Adolesc. Psychopharmacol. 2017, 27, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, L.L.; Swanson, J.M.; Hechtman, L.; Waxmonsky, J.; Arnold, L.E.; Molina, B.S.G.; Hinshaw, S.P.; Jensen, P.S.; Abikoff, H.B.; Wigal, T.; et al. Trajectories of Growth Associated With Long-Term Stimulant Medication in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 978–989. [Google Scholar] [CrossRef]
- Swanson, J.M.; Arnold, L.E.; Molina, B.S.G.; Sibley, M.H.; Hechtman, L.T.; Hinshaw, S.P.; Abikoff, H.B.; Stehli, A.; Owens, E.B.; Mitchell, J.T.; et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: Symptom persistence, source discrepancy, and height suppression. J. Child Psychol. Psychiatry 2017, 58, 663–678. [Google Scholar] [CrossRef]
- Howard, J.T.; Walick, K.S.; Rivera, J.C. Preliminary Evidence of an Association Between ADHD Medications and Diminished Bone Health in Children and Adolescents. J. Pediatric Orthop. 2017, 37, 348–354. [Google Scholar] [CrossRef]
- Lahat, E.; Weiss, M.; Ben-Shlomo, A.; Evans, S.; Bistritzer, T. Bone mineral density and turnover in children with attention-deficit hyperactivity disorder receiving methylphenidate. J. Child Neurol. 2000, 15, 436–439. [Google Scholar] [CrossRef]
- Poulton, A.; Briody, J.; McCorquodale, T.; Melzer, E.; Herrmann, M.; Baur, L.A.; Duque, G. Weight loss on stimulant medication: How does it affect body composition and bone metabolism?—A prospective longitudinal study. Int. J. Pediatric Endocrinol. 2012, 2012, 30. [Google Scholar] [CrossRef]
- Poulton, A.S.; Bui, Q.; Melzer, E.; Evans, R. Stimulant medication effects on growth and bone age in children with attention-deficit/hyperactivity disorder: A prospective cohort study. Int. Clin. Psychopharmacol. 2016, 31, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Feuer, A.J.; Thai, A.; Demmer, R.T.; Vogiatzi, M. Association of Stimulant Medication Use With Bone Mass in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. JAMA Pediatrics 2016, 170, e162804. [Google Scholar] [CrossRef] [PubMed]
- Graham, J. Detecting low bone mineral density from dental radiographs: A mini-review. Clin. Cases Miner. Bone Metab. 2015, 12, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Valerio, C.S.; Trindade, A.M.; Mazzieiro, E.T.; Amaral, T.P.; Manzi, F.R. Use of digital panoramic radiography as an auxiliary means of low bone mineral density detection in post-menopausal women. Dento Maxillo Facial Radiol. 2013, 42, 20120059. [Google Scholar] [CrossRef]
- Calciolari, E.; Donos, N.; Park, J.C.; Petrie, A.; Mardas, N. Panoramic measures for oral bone mass in detecting osteoporosis: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 17s–27s. [Google Scholar] [CrossRef]
- Paulsson-Bjornsson, L.; Adams, J.; Bondemark, L.; Devlin, H.; Horner, K.; Lindh, C. The impact of premature birth on the mandibular cortical bone of children. Osteoporos. Int. 2015, 26, 637–644. [Google Scholar] [CrossRef]
- Apolinario, A.C.; Figueiredo, P.T.; Guimaraes, A.T.; Acevedo, A.C.; Castro, L.C.; Paula, A.P.; Paula, L.M.; Melo, N.S.; Leite, A.F. Pamidronate affects the mandibular cortex of children with osteogenesis imperfecta. J. Dent. Res. 2015, 94, 95s–102s. [Google Scholar] [CrossRef]
- Wren, T.A.; Kalkwarf, H.J.; Zemel, B.S.; Lappe, J.M.; Oberfield, S.; Shepherd, J.A.; Winer, K.K.; Gilsanz, V. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: Persistence of low bone mass to maturity. J. Pediatrics 2014, 164, 1280–1285.e1282. [Google Scholar] [CrossRef]
- Spencer, T.; Biederman, J.; Wilens, T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics 1998, 102, 501–506. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W.J.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 6e: South Asia Edition-E-Book; Elsevier: Mumbai, India, 2019. [Google Scholar]
- Tounta, T.S. Diagnosis of osteoporosis in dental patients. J. Frailty Sarcopenia Falls 2017, 2, 21–27. [Google Scholar] [CrossRef]
- Devlin, H.; Horner, K. Mandibular radiomorphometric indices in the diagnosis of reduced skeletal bone mineral density. Osteoporos. Int. 2002, 13, 373–378. [Google Scholar] [CrossRef]
- Taguchi, A.; Tsuda, M.; Ohtsuka, M.; Kodama, I.; Sanada, M.; Nakamoto, T.; Inagaki, K.; Noguchi, T.; Kudo, Y.; Suei, Y.; et al. Use of dental panoramic radiographs in identifying younger postmenopausal women with osteoporosis. Osteoporos. Int. 2006, 17, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Devlin, H.; Harvey, L. Detecting patients with low skeletal bone mass. J. Dent. 2002, 30, 171–175. [Google Scholar] [CrossRef]
- Tortolani, P.J.; McCarthy, E.F.; Sponseller, P.D. Bone mineral density deficiency in children. J. Am. Acad. Orthop. Surg. 2002, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Nikneshan, S.; Sharafi, M.; Emadi, N. Evaluation of the accuracy of linear and angular measurements on panoramic radiographs taken at different positions. Imaging Sci. Dent. 2013, 43, 191–196. [Google Scholar] [CrossRef]
- Riecke, B.; Friedrich, R.E.; Schulze, D.; Loos, C.; Blessmann, M.; Heiland, M.; Wikner, J. Impact of malpositioning on panoramic radiography in implant dentistry. Clin. Oral Investig. 2015, 19, 781–790. [Google Scholar] [CrossRef]
- Dutra, V.; Susin, C.; da Costa, N.P.; Veeck, E.B.; Bahlis, A.; Fernandes Ada, R. Measuring cortical thickness on panoramic radiographs: A validation study of the Mental Index. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 686–691. [Google Scholar] [CrossRef]

| Variable | ADHD N = 38 | Control N = 48 | p Value * |
|---|---|---|---|
| Age (years) | 12.05 ± 3.24 | 12.76 ± 2.88 | 0.3 |
| Gender | |||
| Male | 26 (68%) | 23 (48%) | 0.056 |
| Female | 12 (32%) | 25 (52%) | |
| Cortical width (mm) | 2.77 ± 0.33 | 3.04 ± 0.46 | 0.004 |
| Cortical width, left (mm) | 2.76 ± 0.36 | 3.07 ± 0.48 | 0.001 |
| Cortical width, right (mm) | 2.78 ± 0.34 | 2.99 ± 0.48 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostiner, H.; Kats, L.; Kot-Limon, N.; Dolev, E.; Blumer, S. Possible Association between Methylphenidate and Mandibular Bone Characteristics Detected by Dental Panoramic Radiograph in Children and Adolescents with ADHD. Children 2022, 9, 1276. https://doi.org/10.3390/children9091276
Kostiner H, Kats L, Kot-Limon N, Dolev E, Blumer S. Possible Association between Methylphenidate and Mandibular Bone Characteristics Detected by Dental Panoramic Radiograph in Children and Adolescents with ADHD. Children. 2022; 9(9):1276. https://doi.org/10.3390/children9091276
Chicago/Turabian StyleKostiner, Hadas, Lazar Kats, Nurit Kot-Limon, Eran Dolev, and Sigalit Blumer. 2022. "Possible Association between Methylphenidate and Mandibular Bone Characteristics Detected by Dental Panoramic Radiograph in Children and Adolescents with ADHD" Children 9, no. 9: 1276. https://doi.org/10.3390/children9091276
APA StyleKostiner, H., Kats, L., Kot-Limon, N., Dolev, E., & Blumer, S. (2022). Possible Association between Methylphenidate and Mandibular Bone Characteristics Detected by Dental Panoramic Radiograph in Children and Adolescents with ADHD. Children, 9(9), 1276. https://doi.org/10.3390/children9091276