Abstract
Consensus on the optimal management of asymptomatic congenital pulmonary airway malformation (CPAM) is lacking, and comparison between studies remains difficult due to a large variety in outcome measures. We aimed to define a core outcome set (COS) for pediatric patients with an asymptomatic CPAM. An online, three-round Delphi survey was conducted in two stakeholder groups of specialized caregivers (surgeons and non-surgeons) in various European centers. Proposed outcome parameters were scored according to level of importance, and the final COS was established through consensus. A total of 55 participants (33 surgeons, 22 non-surgeons) from 28 centers in 13 European countries completed the three rounds and rated 43 outcome parameters. The final COS comprises seven outcome parameters: respiratory insufficiency, surgical complications, mass effect/mediastinal shift (at three time-points) and multifocal disease (at two time-points). The seven outcome parameters included in the final COS reflect the diversity in priorities among this large group of European participants. However, we recommend the incorporation of these outcome parameters in the design of future studies, as they describe measurable and validated outcomes as well as the accepted age at measurement.
1. Introduction
The debate is ongoing on whether asymptomatic congenital pulmonary airway malformation (CPAM) patients require surgical resection versus a conservative follow-up scheme [1,2,3,4]. Those in favor of surgical resection suggest that the risk of recurrent infection or acute respiratory distress can complicate subsequent surgery [3,5]. Furthermore, CPAM lesions have been associated with malignancy, which for some warrants an elective surgical resection [6,7,8]. Those in favor of a conservative follow-up scheme emphasize that the majority of these lesions remain asymptomatic and that some may even regress spontaneously [5,9,10]. Additionally, they state that malignancy is extremely rare and that cases of malignancy have also been described following surgical resection [2,5,11,12]. The disagreement on this subject was already highlighted in 2015 by the simultaneous publication of two opposing articles in Seminars in Pediatric Surgery, one of which advocates surgical resection while the other advocates a conservative follow-up scheme in asymptomatic CPAM [2,3]. Apart from this, multiple studies reflect on the debate surrounding the management of this patient group [13,14,15].
Previous research in CPAM patients examined a wide range of outcome parameters. For example, several studies focused on symptom development in patients that were initially asymptomatic during the neonatal period. The reported proportion ranges from 3% to 64%, although follow-up duration varied from one month to several years [5,10,16,17,18]. Other studies focused on pulmonary function in CPAM patients and tested a variety of techniques, including plethysmography, spirometry and exercise tolerance testing [19,20,21,22,23]. Between these studies, the subjects’ age varied from several months to twelve years, and a comparison between those subjects that underwent surgery and those that were managed conservatively was not universally present. Further studies focused on the presence of pulmonary symptoms or reported the prevalence of certain radiological abnormalities or chest wall deformities during follow-up [10,24,25].
The abovementioned studies are valuable in understanding the natural history of patients with CPAM. However, a reliable comparison between them is complicated by the variety in outcome parameters, measurement methods and subjects’ age.
Selecting appropriate outcome parameters is often the first step towards a uniform design of studies, which in turn will facilitate comparability between interventions. The Core Outcome Measures in Effectiveness Trials (COMET) initiative was developed to guide the process of selecting appropriate outcome parameters, and has already been utilized to develop multiple core outcome sets for a variety of topics [26,27,28,29]. Such a core outcome set has not yet been developed for the management of CPAM, or congenital lung lesions in general. The primary aim of this study was therefore to investigate if defining such a core outcome set for CPAM lesions was feasible among a large group of specialized caregivers in Europe, despite the opposing standpoints between treating specialists.
2. Materials and Methods
This study was performed according to the Delphi consensus methods of the COMET handbook [26]. The previously published study protocol provides a detailed description of the methods [30]. In short, participants were recruited through a pre-existing network of pediatric surgeons and pediatric pulmonologists interested in collaboration concerning the management of CPAM. Potential participants were encouraged to enroll colleagues, who received an email explaining the aims and procedures of the Delphi survey. Three extensive literature reviews were scrutinized for outcome measures, which were formulated to match a standard format: (1) the outcome parameter, (2) the measurement instrument and (3) the age at assessment [2,3,5]. Participants were asked to score every outcome parameter according to the following question: “How important would you rate the following outcome parameter for determining the optimal management of asymptomatic CPAM patients?”. All included outcome parameters focused on postnatal, asymptomatic patients, regardless of the chosen management strategy and the moment during follow-up. During the survey, participants could suggest additional outcome parameters, which were added provided they were indeed original. Participants could also add new time-points for an outcome parameter. A three-round Delphi survey was performed, in which participants had 4 weeks to complete their scoring for each round and received a weekly reminder email. Participants were assigned to either a surgical stakeholder group or a non-surgical group, and were asked to score outcome parameters according to a nine-point Likert scale. This scale consisted of three categories: not important (scoring 1–3), important but not critical (scoring 4–6) and critical (scoring 7–9). Those who had completed the first round were invited to participate in the second round. They were shown their own scoring of the first round as well as the median scoring of their own stakeholder group for all outcome parameters. The instruction was to adapt their own scoring if desirable, and to score the additionally suggested outcome parameters. Preceding the third round, we excluded those outcome parameters that met the following preset exclusion criteria: >70% of the participants rated the outcome as not important (scoring 1–3) and <15% rated it as critical (scoring 7–9). All participants who had completed the second round were invited to participate in the third round, which proceeded in analogy to the second round, except that the median scores of both stakeholder groups were provided and no new outcome parameters were offered. Outcome parameters met the preset criteria for consensus when >70% of the participants rated the outcome 7–9 and <15% rated it 1–3. All outcome parameters that reached consensus were included in the final COS. Finally, all participants were asked to state in their own words which outcome parameter they perceived as the most important in determining the best management of asymptomatic CPAM patients.
Data Analysis
Throughout the Delphi survey, the publicly accessible ‘Welphi’ survey tool was used to record input from participants [31]. Anonymized data were stored on a secure online server and managed according to the European General Data Protection Regulation [32]. Data were analyzed with the statistical package SPSS (version 25, IBM Corp., Armonk, NY, USA). Following the final survey round, the median scoring of each outcome parameter was calculated per stakeholder group and compared between the two groups using the Mann–Whitney U test. Scores for each parameter were divided in three categories: not important (scoring 1–3), important but not critical (scoring 4–6) and critical (scoring 7–9). The percentage of scoring in each category was calculated, after which the final COS was defined according to the preset criteria.
Attrition rate was assessed separately for each round and stakeholder group by calculating the percentage of non-continuing participants, in accordance with the COMET handbook guidelines [26].
To prevent an overestimation of the degree of consensus due to selective drop-out during the survey, attrition bias was tested separately in each stakeholder group for each round of the Delphi survey [33]. For each outcome parameter, the Mann–Whitney U test served to compare average scores between those only completing the round in question and those who also completed the following round.
3. Results
Twenty initial outcome parameters were obtained from the literature review, which were pooled into ten categories: respiratory symptoms, infection, parental anxiety, quality of life, anthropometric measurements, lesion characteristics, spirometry results, exercise tolerance, surgical complications, and malignancy. A flowchart detailing the three-round Delphi survey process is shown in Figure 1.
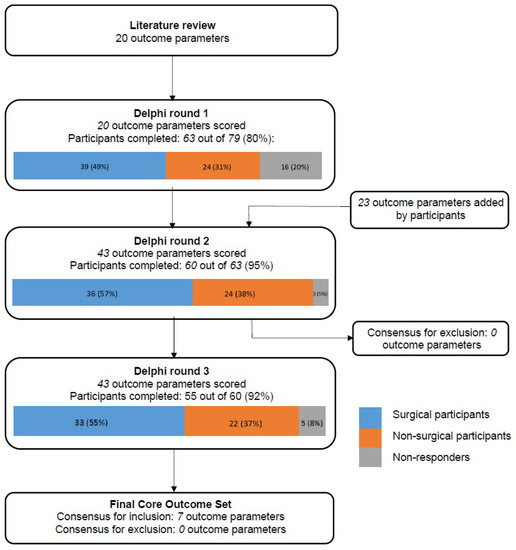
Figure 1.
Flowchart of the 3-round Delphi Survey.
3.1. Round 1
A total of 79 potential participants (45 (57%) surgeons; 34 (43%) non-surgeons) were invited for the first round, of whom 63 (80%) participants completed this round (39 (62%) surgeons; 24 (38%) non-surgeons). A total of 20 outcome parameters were scored in the first round and participants suggested 23 additional parameters. No parameters were excluded after the first round.
3.2. Round 2
All 63 participants who completed the first round were invited for the second round, which was completed by 60 (95%) participants (36 (60%) surgeons; 24 (40%) non-surgeons). A total of 43 outcome parameters were scored or rescored. After the second round, no parameters met the criteria for exclusion; thus, all parameters were carried through to the third round.
3.3. Round 3
All 60 participants who completed the second round were invited for the third round, which was completed by 55 (92%) participants (33 (60%) surgeons; 22 (40%) non-surgeons), representing 28 centers in 13 countries. An overview of all outcome parameters, and their assigned scores, can be found in Table 1. A total of 43 outcome parameters were scored in the third round and 17 parameters across eight domains were scored significantly different between the stakeholder groups.

Table 1.
Summary of outcome parameter scoring per stakeholder group during Delphi survey.
3.4. Final Core Outcome Set
Table 2 shows the final COS. A total of 7 out of the 43 (16%) outcome parameters scored during the final survey round met the criteria for consensus. These outcome parameters were distributed across three domains: respiratory symptoms, surgical complications and lesion characteristics.

Table 2.
Final core outcome set.
Eight outcome parameters met the criteria for consensus in only one of the stakeholder groups, and were therefore not included in the final COS (Table 3). Within the surgical stakeholder group, the following outcome parameters met the criteria for consensus: histology proven malignancy, fever and consolidation on X-ray at the site of the CPAM, and hospital admission with intravenous (IV) antibiotics due to lower respiratory tract infection (LRTI). Within the non-surgical stakeholder group, consensus was achieved on the outcome parameters wheezing (defined as >3 episodes per year), multiple spirometry measurements (FVC, FEF25–75 and FEV1), and evidence of systemic blood supply to the lesion. After completing the final round of the survey, participants were asked to choose one outcome parameter that they found to be the most important for determining the optimal management for asymptomatic CPAM patients, the results of which can be found in Table 4.

Table 3.
Core outcome set per stakeholder group.

Table 4.
Outcome parameters scored as most important for determining the optimal management of asymptomatic CPAM patients.
3.5. Attrition Rate and Attrition Bias
Thirty-three out of the 39 surgeons who completed the first round completed all three rounds, resulting in an attrition rate of 15%. The corresponding figures for the non-surgeons are 22 out of 24, resulting in an attrition rate of 8%. No significant attrition bias was found in either stakeholder group for any of the rounds of the Delphi survey.
4. Discussion
This study aimed to reach consensus on a COS for the management of asymptomatic CPAM patients among a large group of specialized caregivers across Europe. Universal use of standardized outcome parameters in a COS has the potential to increase the quality and relevance of future research in asymptomatic CPAM patients. The online, three-round Delphi survey was completed in 28 specialized medical centers across Europe, which led to consensus for seven outcome parameters: respiratory insufficiency, surgical complications, mass effect/mediastinal shift (at three time-points) and multifocal disease (at two time-points).
An important outcome parameter was respiratory insufficiency requiring supplemental oxygen, ventilation and/or surgical resection, which is unsurprising as this was found to be a major reason to resect a CPAM in a recent European survey [1]. Nevertheless, retrospective studies—with generally limited follow-up—estimated that only 3–24% of initially asymptomatic patients become symptomatic through infancy [5,10,16,34,35]. Long-term follow-up studies in asymptomatic CPAM patients are lacking, preventing accurate estimation of long-term symptom development rate in this patient group. Future research with long-term follow-up is therefore urgently needed. On the other side of symptom development, surgical complications are also included in this COS. Again, a longitudinal comparison between surgical complication rates and the potential burden of conservative follow-up is lacking in literature.
This COS includes radiological abnormalities, namely mediastinal shift and multifocal disease at various ages. In this respect, previous studies have only investigated the correlation between prenatally diagnosed mediastinal shift and perinatal outcome, showing ambiguous results, possibly resulting from the various measurement methods [36,37,38,39]. The relation between mediastinal shift and long-term outcome is yet to be investigated. Somewhat surprisingly, the COS produced in this study does not include outcome parameters directly linked to lesion size, even though several studies found a correlation between lesion size and symptom development [40,41]. However, lesion size can be linked to mediastinal shift to a certain degree, as a large lesion will have a higher chance of causing this shift. Multifocal congenital pulmonary disease, also included in this COS, has been described in several studies in association with a broad differential diagnosis, including bufllae, pneumatoceles, infectious processes, and malignancies such as pleuropulmonary blastoma (PPB) and adenocarcinoma in situ (AIS, formerly known as bronchoalveolar carcinomar (BAC)) [42,43]. The relationship between multifocal disease and the development of symptoms as well as outcome in patients with CPAM remains unclear until now. The relationship between CPAM and malignancy needs further investigation because the exact incidence remains unknown and research of relevant molecular and genetic factors is ongoing [5]. However, particular caution is advised in case the lesion is diagnosed after birth, or in relatively rare cases of CPAM type 4, as these characteristics are associated with a higher risk of malignancy [8,44,45,46].
This study was initiated because of the current heterogeneity in reported outcome parameters and their measurement methods in studies concerning CPAM patients. The final COS confirms this heterogeneity by the differences in outcome parameters between the two stakeholder groups. Outcome parameters such as infection and proven malignancy after resection reached consensus among surgeons. The non-surgeons reached consensus on outcome parameters concerning wheezing, lung function and the radiological assessment of systemic blood supply to the lesion. We hypothesize that surgical specialists consider outcome parameters linked to the indication for surgical resection in CPAM patients most important, while pediatric pulmonologists and neonatologists consider outcome parameters concerning pulmonary morbidity and radiological assessment more important, resulting in a slight bias per stakeholder group. The differences in scoring between the two stakeholder groups highlight the impact of multidisciplinary care for CPAM patients and emphasize the importance of taking all different perspectives into consideration in the design of future studies.
The outcome parameters included in the final COS do not fully overlap with those mentioned by participants as most important for determining the optimal management for asymptomatic CPAM patients. For example, while the final COS does not include infection-related outcome parameters, infection-related outcome was provided as the most important by 32% of participants, the most often of all outcome categories. The opposite was true for the outcome parameter of respiratory insufficiency, which achieved the highest level of consensus and is included in the final COS, while only three participants (6%) scored it as the most important outcome parameter. These dissimilarities, again, confirm the diversity in opinions among specialist caregivers concerning the management of asymptomatic CPAM patients.
To our knowledge, this is the first international Delphi survey on the management of asymptomatic CPAM patients. A valuable strength of this study is the large number of participants that completed the three-round survey: 55 representatives from 28 specialized centers spread across Europe. The design of this three-round survey proved to function according to the preset criteria, and attrition rate and attrition bias were both within limits. In other words, the drop-out rate during the survey was acceptable and there was no selective drop-out of participants with a certain (minority) opinion.
The lack of patient and public involvement in the development of the final COS is a limitation of this study. The most important consideration here was the knowledge that established patient societies or patient-support groups are scarce and widespread, making it challenging to establish an unbiased general opinion on the most important outcome parameters for this group [47]. Furthermore, we imagine that parents of asymptomatic patients might have difficulty providing input on desired outcome measures, as the large majority will not be confronted with symptoms or complications in their children [30].
Another limitation of this study can be found in the merging of the input from both stakeholder groups into one shared core outcome set, leading to the loss of certain outcome parameters that scored high in only one of the two stakeholder groups. However, in our opinion, this situation quite accurately reflects the daily clinical practice, in which multidisciplinary teams decide on the management of patients with congenital lung disease. Lastly, the Delphi survey was completed by more surgical specialists than non-surgical specialists (60% vs. 40%), possibly influencing the composition of the final core outcome set.
The seven outcome parameters included in this final COS can be applied for the development of prospective studies as they describe measurable and validated outcomes as well as the accepted age at measurement. For that matter, each of the outcome parameters could be included individually in a tailor-made study design. We believe that a prospective randomized study, including a long-term follow-up period, is necessary to evaluate whether surgical resection or a conservative follow-up is superior for asymptomatic CPAM patients. Furthermore, we suggest that in addition to this COS, functional outcome parameters such as endurance tests or lung function tests should be taken into consideration, as they are especially suitable for comparing outcomes in a generally asymptomatic population. Lastly, as a means to consider multiple perspectives and maximize the implication of future findings, we suggest patient and public involvement in the design of future studies in this population.
In conclusion, this study delivers the first COS intended to evaluate the management of asymptomatic CPAM patients. It reflects the diversity in priorities among the large group of European participants that contributed to the development of the COS. Consequently, the core outcome set is not a ready-to-use template for the design of future studies, though it does accurately highlight relevant individual outcome parameters. We recommend researchers to incorporate these outcome parameters in the design of future studies, as this will test the practicality—and possibly lead to the optimization—of this COS.
Author Contributions
C.M.K., S.M.H. and J.M.S. contributed to conceptualization and design of the study, acquired data, carried out analyses and interpreted the data. Furthermore, they prepared the original draft article, revised it for important intellectual content and approved the final version for publication. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. D.M., N.M., N.C., S.G., P.B., C.M.B., A.S., S.H., H.T., J.S., M.S.(Maarten Schurink), L.D., P.L., H.S., S.T.-L., M.M., A.B., R.S., M.S. (Michael Singh), I.Y., N.R.V.M.R.-M., C.K.v.d.E., N.Q., D.W.C., R.P., M.A.G.E.B., L.W., M.P., M.S. (Michael Stanton), E.H., M.Z., F.M., H.A.W.M.T., R.M.H.W. and J.M.S. contributed to conception and design of the study, revised the draft version for important intellectual content and gave approval of the final version for publication. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the anonymous handling of data from participants in this survey.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
We thank Ko Hagoort for critically reviewing the manuscript and providing editorial advice. Collaborators: The following medical specialists initiated the CONNECT study consortium: S.M. Hermelijn, C.M. Kersten, R.M.H. Wijnen, H.A.W.M. Tiddens, J.M. Schnater (Erasmus MC Sophia Children’s Hospital, Rotterdam, the Netherlands). The following medical specialists form the CONNECT study consortium COS development group (in alphabetical order per center): Ian Sinha (Alder Hey Children’s Hospital, Liverpool, United Kingdom); S.A. de Beer, E. Haarman, S. Terheggen-Lagro (Amsterdam University Medical Centre, Amsterdam, the Netherlands); N. Cobanoglu, G. Gollu (Ankara University, School of Medicine, Ankara, Turkey); Pietro Bagolan, (Bambino Gesù Pediatric Hospital, “Tor Vergata” University, Rome, Italy); F. Morini (Azienda Ospedaliero—Universitaria Meyer, Florence, Italy); M. Singh (Birmingham Children’s Hospital, Birmingham, United Kingdom); D.W. Cox (Children’s Health Ireland at Crumlin, Dublin, Ireland); I. Yardley (Evelina London Children’s Hospital, London, United Kingdom); S.C.M. Cochius—den Otter (Erasmus MC Sophia Children’s Hospital, Rotterdam, the Netherlands); L. Desender, H. Schaballie (Ghent University Hospital, Ghent, Belgium); D. Mullassery, N. Muthialu, C. Wallis (Great Ormond Street Hospital, London, United Kingdom); L. Martelius, M. Pakarinen, J. Suominen (Helsinki Children’s Hospital, Helsinki, Finland); R. Sfeir (Hopital Jeanne de Flandre, Lille, France); A. Bonnard (Hopital Universitaire Robert Debré, Paris, France); S. Gartner, A. Lain (Hospital Universitari Vall d´Hebron, Barcelona, Spain); P. Losty (Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom); C.Y. Bitkover, E. Caffrey-Osvald, P. Conner, C. Mesas Burgos, (Karolinska Institutet, Stockholm, Sweden); E. Hannon (Leeds Children’s Hospital, Leeds, United Kingdom); N.R.V.M. Rikkers-Mutsaerts, I. de Vreede (Leiden University Medical Centre, Leiden, the Netherlands); M.A.G.E. Bannier, Q. Jobsis (Maastricht University Medical Centre, Maastricht, the Netherlands); G. Singer, H. Till (Medical University of Graz, Graz, Austria); M. Metzelder, P Sezen (Medizinische Universität Wien, Vienna, Austria); N. Qvist (Odense Univeristy Hospital, Odense, Denmark); G. Aksnes, K. Ertresvåg (Oslo University Hospital, Oslo, Norway); H. Steyaert (Queen Fabiola Children’s University Hospital, Brussels, Belgium); S. van der Heide, J. Roukema, M. Schurink (Radboud University Medical Centre, Amalia Children’s Hospital, Nijmegen, the Netherlands); M. Zampoli (Red Cross Children’s Hospital, Cape Town, South Africa); R. Peters (Royal Manchester Children’s Hospital, Manchester, United Kingdom); M. Boon, H. Decaluwé, J. Deprest, M. Proesmans (University Hospital Leuven, Leuven, Belgium); T. Schaible, L. Wessel, K. Zahn, (University Hospital Mannheim, Mannheim, Germany); M. Stanton (University Hospital Southampton, Southampton, United Kingdom); P. Gamba, A. Sgrò (University Hospital Padua, Padua, Italy); C. K. van der Ent, K.M. Winter-de Groot (University Medical Centre Utrecht, Utrecht, the Netherlands); S. Heyman, D. Vervloessem (ZNA-GZA Paola Children’s Hospital, Antwerp, Belgium).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morini, F.; Zani, A.; Conforti, A.; van Heurn, E.; Eaton, S.; Puri, P.; Rintala, R.; Lukac, M.; Kuebler, J.F.; Friedmacher, F.; et al. Current Management of Congenital Pulmonary Airway Malformations: A “European Pediatric Surgeons’ Association” Survey. Eur. J. Pediatr. Surg. 2018, 28, 1–5. [Google Scholar] [CrossRef]
- Stanton, M. The argument for a non-operative approach to asymptomatic lung lesions. Semin. Pediatr. Surg. 2015, 24, 183–186. [Google Scholar] [CrossRef]
- Singh, R.; Davenport, M. The argument for operative approach to asymptomatic lung lesions. Semin. Pediatr. Surg. 2015, 24, 187–195. [Google Scholar] [CrossRef]
- Hermelijn, S.M.; Elders, B.B.L.J.; Ciet, P.; Wijnen, R.M.H.; Tiddens, H.; Schnater, J.M. A clinical guideline for structured assessment of CT-imaging in congenital lung abnormalities. Paediatr. Respir. Rev. 2021, 37, 80–88. [Google Scholar] [CrossRef]
- Stanton, M.; Njere, I.; Ade-Ajayi, N.; Patel, S.; Davenport, M. Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J. Pediatr. Surg. 2009, 44, 1027–1033. [Google Scholar] [CrossRef]
- MacSweeney, F.; Papagiannopoulos, K.; Goldstraw, P.; Sheppard, M.N.; Corrin, B.; Nicholson, A.G. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am. J. Surg. Pathol. 2003, 27, 1139–1146. [Google Scholar] [CrossRef]
- Baird, R.; Puligandla, P.S.; Laberge, J.M. Congenital lung malformations: Informing best practice. Semin. Pediatr. Surg. 2014, 23, 270–277. [Google Scholar] [CrossRef]
- Kunisaki, S.M.; Lal, D.R.; Saito, J.M.; Fallat, M.E.; St Peter, S.D.; Fox, Z.D.; Heider, A.; Chan, S.S.; Boyd, K.P.; Burns, R.C.; et al. Pleuropulmonary Blastoma in Pediatric Lung Lesions. Pediatrics 2021, 147, e2020028357. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chao, A.S.; Chang, Y.L.; Kuo, D.M.; Hsieh, T.T.; Hung, H.T. Outcome of congenital cystic adenomatoid malformation of the lung after antenatal diagnosis. Int. J. Gynecol. Obstet. 2005, 89, 99–102. [Google Scholar] [CrossRef]
- Cook, J.; Chitty, L.S.; De Coppi, P.; Ashworth, M.; Wallis, C. The natural history of prenatally diagnosed congenital cystic lung lesions: Long-term follow-up of 119 cases. Arch. Dis Child. 2017, 102, 798–803. [Google Scholar] [CrossRef]
- Benjamin, D.R.; Cahill, J.L. Bronchioloalveolar carcinoma of the lung and congenital cystic adenomatoid malformation. Am. J. Clin. Pathol. 1991, 95, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Papagiannopoulos, K.A.; Sheppard, M.; Bush, A.P.; Goldstraw, P. Pleuropulmonary blastoma: Is prophylactic resection of congenital lung cysts effective? Ann. Thorac. Surg. 2001, 72, 604–605. [Google Scholar] [CrossRef]
- Peters, R.T.; Burge, D.M.; Marven, S.S. Congenital lung malformations: An ongoing controversy. Ann. R. Coll. Surg. Engl. 2013, 95, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Eber, E. Antenatal diagnosis of congenital thoracic malformations: Early surgery, late surgery, or no surgery? Semin. Respir. Crit. Care Med. 2007, 28, 355–366. [Google Scholar] [CrossRef]
- David, M.; Lamas-Pinheiro, R.; Henriques-Coelho, T. Prenatal and Postnatal Management of Congenital Pulmonary Airway Malformation. Neonatology 2016, 110, 101–115. [Google Scholar] [CrossRef]
- Kantor, N.; Wayne, C.; Nasr, A. Symptom development in originally asymptomatic CPAM diagnosed prenatally: A systematic review. Pediatr. Surg. Int. 2018, 34, 613–620. [Google Scholar] [CrossRef]
- Criss, C.N.; Musili, N.; Matusko, N.; Baker, S.; Geiger, J.D.; Kunisaki, S.M. Asymptomatic congenital lung malformations: Is nonoperative management a viable alternative? J. Pediatr. Surg. 2018, 53, 1092–1097. [Google Scholar] [CrossRef]
- Kapralik, J.; Wayne, C.; Chan, E.; Nasr, A. Surgical versus conservative management of congenital pulmonary airway malformation in children: A systematic review and meta-analysis. J. Pediatr. Surg. 2016, 51, 508–512. [Google Scholar] [CrossRef]
- Tocchioni, F.; Lombardi, E.; Ghionzoli, M.; Ciardini, E.; Noccioli, B.; Messineo, A. Long-term lung function in children following lobectomy for congenital lung malformation. J. Pediatr. Surg. 2017, 52, 1891–1897. [Google Scholar] [CrossRef]
- Spoel, M.; van de Ven, K.P.; Tiddens, H.A.W.M.; Hop, W.C.J.; Wijnen, R.M.H.; Tibboel, D.; Ijsselstijn, H. Lung function of infants with congenital lung lesions in the first year of life. Neonatology 2013, 103, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Hijkoop, A.; van Schoonhoven, M.M.; van Rosmalen, J.; Tibboel, D.; van der Cammen-van Zijp, M.H.M.; Pijnenburg, M.W.; Cohen-Overbeek, T.E.; Schnater, J.M.; IJsselstijn, H. Lung function, exercise tolerance, and physical growth of children with congenital lung malformations at 8 years of age. Pediatr. Pulmonol. 2019, 54, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Naito, Y.; Beres, A.; Lapidus-Krol, E.; Ratjen, F.; Langer, J.C. Does earlier lobectomy result in better long-term pulmonary function in children with congenital lung anomalies? A prospective study. J. Pediatr. Surg. 2012, 47, 852–856. [Google Scholar] [CrossRef]
- Dincel, A.; Yilmaz Yegit, C.; Ergenekon, A.P.; Erdem Eralp, E.; Gokdemir, Y.; Kiyan, G.; Karadag, B. Long-term respiratory outcomes of post-op congenital lung malformations. Pediatr. Int. 2020, 63, 704–709. [Google Scholar] [CrossRef]
- Calzolari, F.; Braguglia, A.; Valfrè, L.; Dotta, A.; Bagolan, P.; Morini, F. Outcome of infants operated on for congenital pulmonary malformations. Pediatr. Pulmonol. 2016, 51, 1367–1372. [Google Scholar] [CrossRef]
- Pinter, A.; Kalman, A.; Karsza, L.; Verebely, T.; Szemledy, F. Long-term outcome of congenital cystic adenomatoid malformation. Pediatr. Surg. Int. 1999, 15, 332–335. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.M.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET Handbook: Version 1.0. Trials 2017, 18, 280. [Google Scholar] [CrossRef] [Green Version]
- Knaapen, M.; Hall, N.J.; van der Lee, J.H.; Butcher, N.J.; Offringa, M.; Van Heurn, E.W.E.; Bakx, R.; Gorter, R.R. Establishing a core outcome set for treatment of uncomplicated appendicitis in children: Study protocol for an international Delphi survey. BMJ Open 2019, 9, e028861. [Google Scholar] [CrossRef] [Green Version]
- Sherratt, F.C.; Eaton, S.; Walker, E.; Beasant, L.; Blazeby, J.M.; Young, B.; Crawley, E.; Wood, W.W.; Hall, N.J. Development of a core outcome set to determine the overall treatment success of acute uncomplicated appendicitis in children: A study protocol. BMJ Paediatr Open 2017, 1, e000151. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, B.; Ringrow, S.; Clarke, M.; Marshall, J.C.; Connolly, B.; Rose, L.; McAuley, D.F. A Core Outcome Set for Critical Care Ventilation Trials. Crit. Care Med. 2019, 47, 1324–1331. [Google Scholar] [CrossRef]
- Hermelijn, S.; Kersten, C.; Mullassery, D.; Muthialu, N.; Cobanoglu, N.; Gartner, S.; Bagolan, P.; Mesas Burgos, C.; Sgro, A.; Heyman, S.; et al. Development of a core outcome set for congenital pulmonary airway malformations: Study protocol of an international Delphi survey. BMJ Open 2021, 11, e044544. [Google Scholar] [CrossRef]
- Eyes, D. Welphi-Home. Available online: https://www.welphi.com/en/Home.html (accessed on 16 February 2021).
- de Magalhães, S.T. The European Union’s General Data Protection Regulation (GDPR). Available online: https://www.semanticscholar.org/paper/The-European-Union%E2%80%99s-General-Data-Protection-(GDPR)-Magalhaes/6e9933ee4eb634ba691903ccbc83c9abd871747b (accessed on 16 February 2021).
- Sinha, I.P.; Smyth, R.L.; Williamson, P.R. Using the Delphi technique to determine which outcomes to measure in clinical trials: Recommendations for the future based on a systematic review of existing studies. PLoS Med. 2011, 8, e1000393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvat, F.; Michel, J.L.; Benachi, A.; Emond, S.; Revillon, Y. Management of asymptomatic neonatal cystic adenomatoid malformations. J. Pediatr. Surg. 2003, 38, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.; Warne, S.A.; Cacciaguerra, S.; Patel, S.; Greenough, A.; Nicolaides, K. Current outcome of antenally diagnosed cystic lung disease. J. Pediatr. Surg. 2004, 39, 549–556. [Google Scholar] [CrossRef]
- Miller, J.A.; Corteville, J.E.; Langer, J.C. Congenital cystic adenomatoid malformation in the fetus: Natural history and predictors of outcome. J. Pediatr. Surg. 1996, 31, 805–808. [Google Scholar] [CrossRef]
- Bunduki, V.; Ruano, R.; da Silva, M.M.; Miguelez, J.; Miyadahira, S.; Maksoud, J.G.; Zugaib, M. Prognostic factors associated with congenital cystic adenomatoid malformation of the lung. Prenat. Diagn. 2000, 20, 459–464. [Google Scholar] [CrossRef]
- Mallmann, M.R.; Graham, V.; Rösing, B.; Gottschalk, I.; Müller, A.; Gembruch, U.; Geipel, A.; Berg, C. Thoracoamniotic Shunting for Fetal Hydrothorax: Predictors of Intrauterine Course and Postnatal Outcome. Fetal Diagn. Ther. 2017, 41, 58–65. [Google Scholar] [CrossRef]
- Shulman, R.; Sparks, T.N.; Gosnell, K.; Blat, C.; Norton, M.E.; Lee, H.; Gonzalez-Velez, J.; Goldstein, R.B. Fetal Congenital Pulmonary Airway Malformation: The Role of an Objective Measurement of Cardiomediastinal Shift. Am. J. Perinatol. 2019, 36, 225–232. [Google Scholar]
- Aziz, D.; Langer, J.C.; Tuuha, S.E.; Ryan, G.; Ein, S.H.; Kim, P.C. Perinatally diagnosed asymptomatic congenital cystic adenomatoid malformation: To resect or not? J. Pediatr. Surg. 2004, 39, 329–334. [Google Scholar] [CrossRef]
- Hermelijn, S.M.; Dragt, O.V.; Bosch, J.J.; Hijkoop, A.; Riera, L.; Ciet, P.; Wijnen, R.M.H.; Schnater, J.M.; Tiddens, H. Congenital lung abnormality quantification by computed tomography: The CLAQ method. Pediatr. Pulmonol. 2020, 55, 3152–3161. [Google Scholar] [CrossRef]
- Ryu, J.H.; Tian, X.; Baqir, M.; Xu, K. Diffuse cystic lung diseases. Front. Med. 2013, 7, 316–327. [Google Scholar] [CrossRef]
- Priest, J.R.; Williams, G.M.; Hill, D.A.; Dehner, L.P.; Jaffé, A. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr. Pulmonol. 2009, 44, 14–30. [Google Scholar] [CrossRef]
- Nasr, A.; Himidan, S.; Pastor, A.C.; Taylor, G.; Kim, P.C. Is congenital cystic adenomatoid malformation a premalignant lesion for pleuropulmonary blastoma? J. Pediatr. Surg. 2010, 45, 1086–1089. [Google Scholar] [CrossRef]
- Oliveira, C.; Himidan, S.; Pastor, A.C.; Nasr, A.; Manson, D.; Taylor, G.; Yanchar, N.L.; Brisseau, G.; Kim, P.C. Discriminating preoperative features of pleuropulmonary blastomas (PPB) from congenital cystic adenomatoid malformations (CCAM): A retrospective, age-matched study. Eur. J. Pediatr. Surg. 2011, 21, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, C.; Baron, M.; Desselas, E.; Phan, M.H.; Rybak, A.; Thouvenin, G.; Lauby, C.; Irtan, S. Congenital pulmonary airway malformations: State-of-the-art review for pediatrician′s use. Eur. J. Pediatr. 2017, 176, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Davies, A.; Young, A.E. Systematic review of international Delphi surveys for core outcome set development: Representation of international patients. BMJ Open 2020, 10, e040223. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).