Development of an International Database for a Rare Genetic Disorder: The MECP2 Duplication Database (MDBase)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
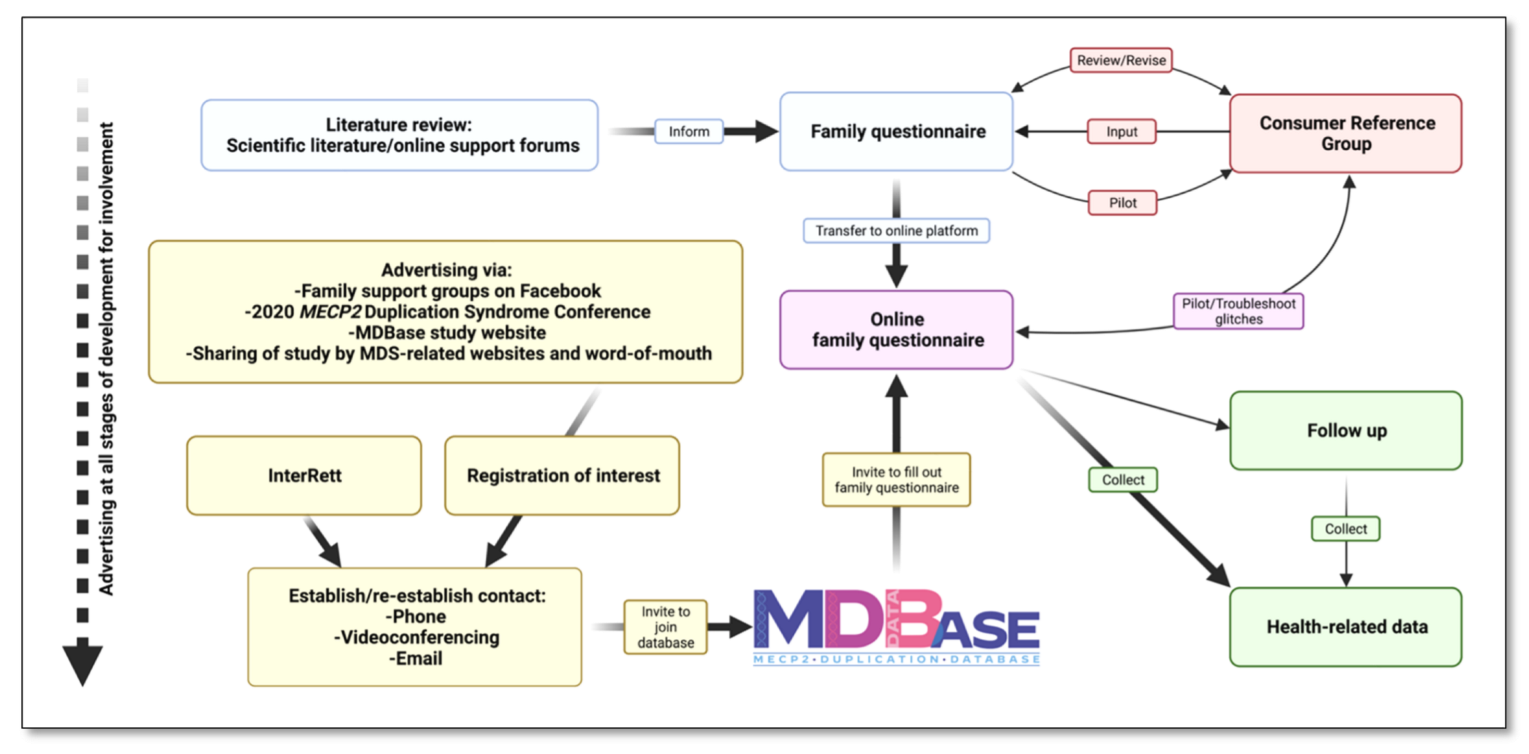
2.2. Formation of a Consumer Reference Group (CRG) and Identification of Key Research Areas
2.3. Drafting a Family Questionnaire
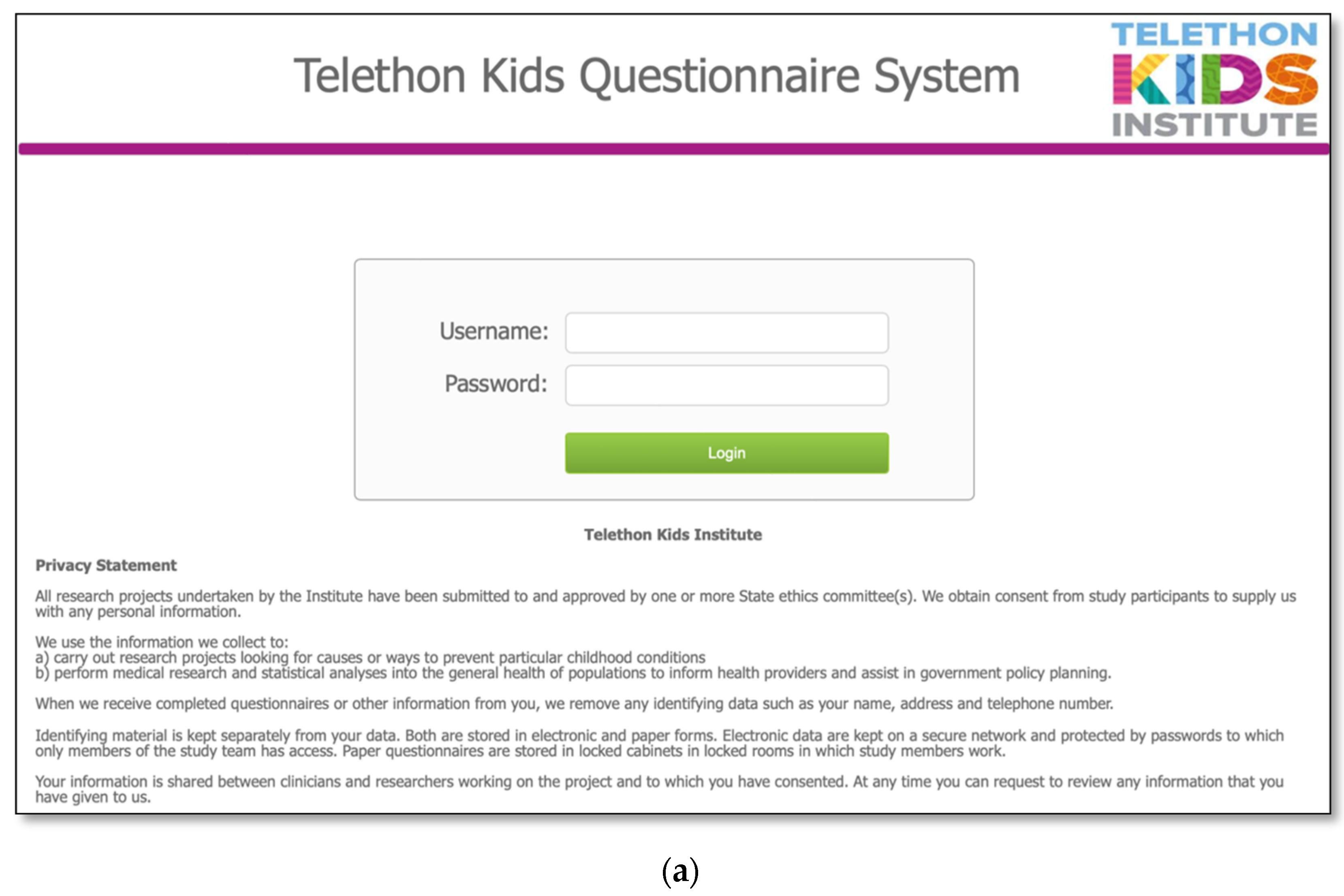
2.4. Database and Online Questionnaire Design
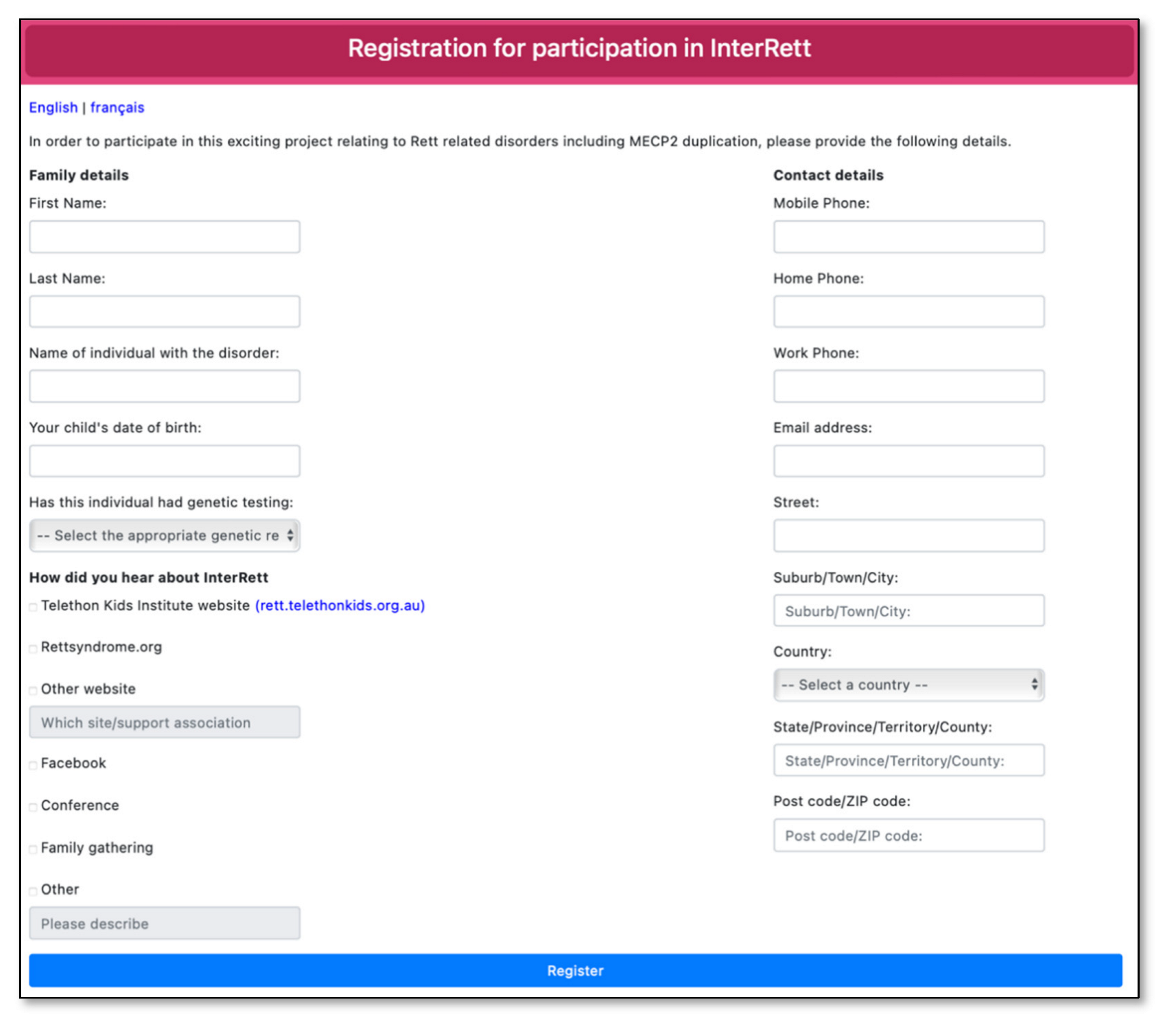
2.5. Advertising, Recruitment and Data Collection
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moliner, A.M.; Waligora, J. The European Union Policy in the Field of Rare Diseases. Adv. Exp. Med. Biol. 2017, 1031, 561–587. [Google Scholar] [PubMed]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crooke, S.T. A call to arms against ultra-rare diseases. Nat. Biotechnol. 2021, 39, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Ta, D.; Downs, J.; Baynam, G.; Wilson, A.; Richmond, P.; Leonard, H. A brief history of MECP2 duplication syndrome: 20-years of clinical understanding. Orphanet J. Rare Dis. 2022, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Lubs, H.; Abidi, F.; Bier, J.A.; Abuelo, D.; Ouzts, L.; Voeller, K.; Fennell, E.; Stevenson, R.E.; Schwartz, C.E.; Arena, F. XLMR syndrome characterized by multiple respiratory infections, hypertelorism, severe CNS deterioration and early death localizes to distal Xq28. Am. J. Med. Genet. 1999, 85, 243–248. [Google Scholar] [CrossRef]
- Giudice-Nairn, P.; Downs, J.; Wong, K.; Wilson, D.; Ta, D.; Gattas, M.; Amor, D.; Thompson, E.; Kirrali-Borri, C.; Ellaway, C.; et al. The incidence, prevalence and clinical features of MECP2 duplication syndrome in Australian children. J. Paediatr. Child. 2019, 55, 1315–1322. [Google Scholar] [CrossRef]
- Miguet, M.; Faivre, L.; Amiel, J.; Nizon, M.; Touraine, R.; Prieur, F.; Pasquier, L.; Lefebvre, M.; Thevenon, J.; Dubourg, C.; et al. Further delineation of the MECP2 duplication syndrome phenotype in 59 French male patients, with a particular focus on morphological and neurological features. J. Med. Genet. 2018, 55, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.; Downs, J.; Russell, A.; Wang, W. Impact of biobanks on research outcomes in rare diseases: A systematic review. Orphanet J. Rare Dis. 2018, 13, 202. [Google Scholar] [CrossRef]
- Rett, A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med. Wochenschr. 1966, 116, 723–726. [Google Scholar]
- Leonard, H.; Cobb, S.; Downs, J. Clinical and biological progress over 50 years in Rett syndrome. Nat. Rev. Neurol. 2017, 13, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurvick, C.L.; De Klerk, N.; Bower, C.; Christodoulou, J.; Ravine, D.; Ellaway, C.; Williamson, S.; Leonard, H. Rett syndrome in Australia: A review of the epidemiology. J. Pediatr. 2006, 148, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Wong, K.; Jacoby, P.; Downs, J.; Leonard, H. Twenty years of surveillance in Rett syndrome: What does this tell us? Orphanet J. Rare Dis. 2014, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bebbington, A.; Anderson, A.; Ravine, D.; Fyfe, S.; Pineda, M.; De Klerk, N.; Ben-Zeev, B.; Yatawara, N.; Percy, A.; Kaufmann, W.E.; et al. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology 2008, 70, 868–875. [Google Scholar] [CrossRef]
- Fehr, S.; Wilson, M.; Downs, J.; Williams, S.; Murgia, A.; Sartori, S.; Vecchi, M.; Ho, G.; Polli, R.; Psoni, S.; et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur. J. Hum. Genet. 2013, 21, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Fehr, S.; Leonard, H.; Ho, G.; Williams, S.; de Klerk, N.; Forbes, D.; Christodoulou, J.; Downs, J. There is variability in the attainment of developmental milestones in the CDKL5 disorder. J. Neurodev. Disord. 2015, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Fehr, S.; Downs, J.; Ho, G.; de Klerk, N.; Forbes, D.; Christodoulou, J.; Williams, S.; Leonard, H. Functional abilities in children and adults with the CDKL5 disorder. Am. J. Med. Genet. A 2016, 170, 2860–2869. [Google Scholar] [CrossRef]
- Fehr, S.; Wong, K.; Chin, R.; Williams, S.; de Klerk, N.; Forbes, D.; Krishnaraj, R.; Christodoulou, J.; Downs, J.; Leonard, H. Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology 2016, 87, 2206–2213. [Google Scholar] [CrossRef]
- Lim, Z.; Downs, J.; Wong, K.; Ellaway, C.; Leonard, H. Expanding the clinical picture of the MECP2 duplication syndrome. Clin. Genet. 2017, 91, 557–563. [Google Scholar] [CrossRef]
- Louise, S.; Fyfe, S.; Bebbington, A.; Bahi-Buisson, N.; Anderson, A.; Pineda, M.; Percy, A.; Zeev, B.B.; Wu, X.R.; Bao, X.; et al. InterRett, a model for international data collection in a rare genetic disorder. Res. Autism Spectr. Disord. 2009, 3, 639–659. [Google Scholar] [CrossRef] [Green Version]
- Leonard, H.; Downs, J.; Benke, T.A.; Swanson, L.; Olson, H.; Demarest, S. CDKL5 deficiency disorder: Clinical features, diagnosis, and management. Lancet Neurol. 2022, 21, 563–576. [Google Scholar] [CrossRef]
- MacKay, C.I.; Wong, K.; Demarest, S.T.; Benke, T.A.; Downs, J.; Leonard, H. Exploring genotype-phenotype relationships in the CDKL5 deficiency disorder using an international dataset. Clin. Genet. 2021, 99, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Concannon, T.W.; Fuster, M.; Saunders, T.; Patel, K.; Wong, J.B.; Leslie, L.K.; Lau, J. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J. Gen. Intern. Med. 2014, 29, 1692–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ta, D.; Downs, J.; Baynam, G.; Wilson, A.; Richmond, P.; Leonard, H. Medical Comorbidities in MECP2 Duplication Syndrome: Results from the International MECP2 Duplication Database. Children 2022, 9, 633. [Google Scholar] [CrossRef]
- Leonard, H.; Gold, W.; Samaco, R.; Sahin, M.; Benke, T.; Downs, J. Improving clinical trial readiness to accelerate development of new therapeutics for Rett syndrome. Orphanet J. Rare Dis. 2022, 17, 108. [Google Scholar] [CrossRef]
- Bain, J.M.; Ardalan, A.; Goldman, S. Deliberate paradigm shift in research in rare neurodevelopmental disorders. Orphanet J. Rare Dis. 2021, 16, 263. [Google Scholar] [CrossRef]
- Tones, M.; Cross, M.; Simons, C.; Napier, K.R.; Hunter, A.; Bellgard, M.I.; Heussler, H. Research protocol: The initiation, design and establishment of the Global Angelman Syndrome Registry. J. Intellect. Disabil. Res. 2018, 62, 431–443. [Google Scholar] [CrossRef]
- Bohonowych, J.; Miller, J.; McCandless, S.E.; Strong, T.V. The Global Prader-Willi Syndrome Registry: Development, Launch, and Early Demographics. Genes 2019, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Vrana-Diaz, C.J.; Balasubramanian, P.; Kayadjanian, N.; Bohonowych, J.; Strong, T.V. Variability and change over time of weight and BMI among adolescents and adults with Prader-Willi syndrome: A 6-month text-based observational study. Orphanet J. Rare Dis. 2020, 15, 233. [Google Scholar] [CrossRef]
- Kayadjanian, N.; Vrana-Diaz, C.; Bohonowych, J.; Strong, T.V.; Morin, J.; Potvin, D.; Schwartz, L. Characteristics and relationship between hyperphagia, anxiety, behavioral challenges and caregiver burden in Prader-Willi syndrome. PLoS ONE 2021, 16, e0248739. [Google Scholar] [CrossRef] [PubMed]
- Peleggi, A.; Bohonowych, J.; Strong, T.; Schwartz, L.; Kim, S.-J. Suicidality in individuals with Prader-Willi syndrome: A review of registry survey data. BMC Psychiatry 2021, 21, 438. [Google Scholar] [CrossRef] [PubMed]
- Shelkowitz, E.; Gantz, M.G.; Ridenour, T.A.; Scheimann, A.O.; Strong, T.; Bohonowych, J.; Duis, J. Neuropsychiatric features of Prader-Willi syndrome. Am. J. Med. Genet. A 2022, 188, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Matesevac, L.; Miller, J.L.; McCandless, S.E.; Malloy, J.L.; Bohonowych, J.E.; Vrana-Diaz, C.; Strong, T.V. Thrombosis Risk History and D-dimer Levels in Asymptomatic Individuals with Prader–Willi Syndrome. J. Clin. Med. 2022, 11, 2040. [Google Scholar] [CrossRef]
- Bohonowych, J.E.; Vrana-Diaz, C.J.; Miller, J.L.; McCandless, S.E.; Strong, T.V. Incidence of strabismus, strabismus surgeries, and other vision conditions in Prader-Willi syndrome: Data from the Global Prader-Willi Syndrome Registry. BMC Ophthalmol. 2021, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Shoubridge, C.; Fullston, T.; Gécz, J. ARX spectrum disorders: Making inroads into the molecular pathology. Hum. Mutat. 2010, 31, 889–900. [Google Scholar] [CrossRef]
- Marques Pereira, P.; Schneider, A.; Pannetier, S.; Heron, D.; Hanauer, A. Coffin–Lowry syndrome. Eur. J. Hum. Genet. 2010, 18, 627–633. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Rahman, N. Sotos syndrome. Eur. J. Hum. Genet. 2007, 15, 264–271. [Google Scholar] [CrossRef]
- Karaman, B.; Kayserili, H.; Ghanbari, A.; Uyguner, Z.O.; Toksoy, G.; Altunoglu, U.; Basaran, S. Pallister-Killian syndrome: Clinical, cytogenetic and molecular findings in 15 cases. Mol. Cytogenet. 2018, 11, 45. [Google Scholar] [CrossRef]
- Milani, D.; Manzoni, F.M.; Pezzani, L.; Ajmone, P.; Gervasini, C.; Menni, F.; Esposito, S. Rubinstein-Taybi syndrome: Clinical features, genetic basis, diagnosis, and management. Ital. J. Pediatrics 2015, 41, 4. [Google Scholar] [CrossRef] [Green Version]
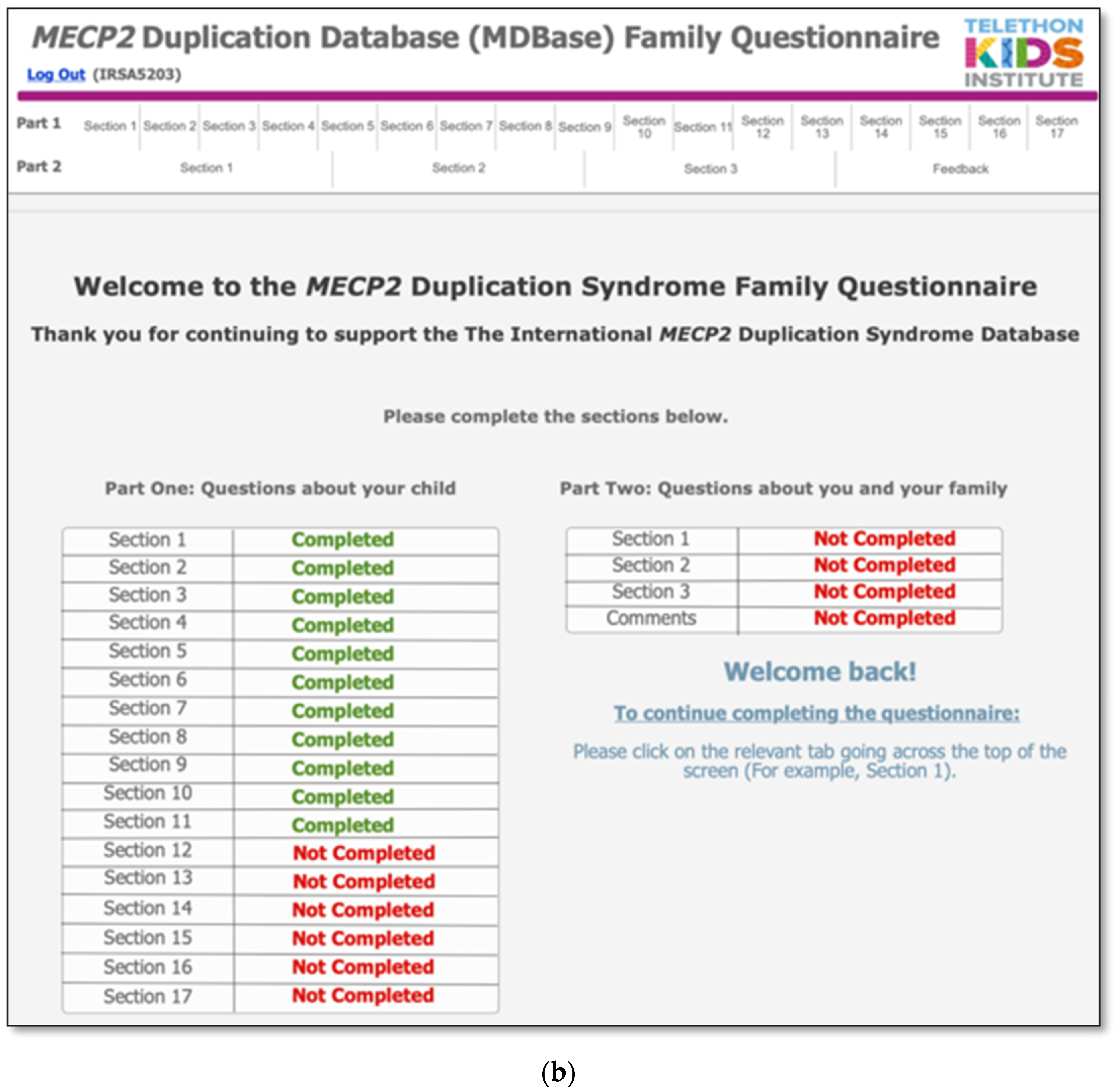

| Part 1: Questions About Individuals with MECP2 Duplication Syndrome (MDS) |
| Section 1: Early experiences |
| Relating to pregnancy and period following birth of individual with MDS, first concerns, and diagnostic tests and examination surrounding MDS diagnosis |
| Section 2: Early development |
| Relating to gross motor, communication and social/emotional, and hand function developmental milestones |
| Section 3: Regression |
| Relating to the loss of any previously learned developmental skill |
| Section 4: Current function |
| Relating to current gross motor, communication, and hand function abilities |
|
| Section 5: Cardiopulmonary |
| Relating to conditions affecting the heart, lungs, and autonomic nervous system |
| Section 6: Epilepsy |
| Relating to seizures and/or epilepsy and their treatment |
| Section 7: Gastrointestinal health |
| Relating to gastrointestinal problems, feeding and surgical interventions |
| Section 8: Ear, nose and throat (ENT) |
| Relating to ear, nose and throat conditions and their treatment |
| Section 9: Other medical conditions |
| Relating to other medical conditions such scoliosis and bone fractures, urinary tract problems, and pain sensitivity |
| Section 10: Sleep |
| Relating to sleeping characteristics |
|
| Section 11: Behaviours |
| Relating to hand stereotypies, bruxism and other behaviours such as Rett syndrome-like and autism-like behaviours |
|
| Section 12: Puberty |
| Relating to sexual development |
| Section 13: Medications |
| Relating to medication history (prescribed, over the counter and natural medications as well as supplements) |
| Section 14: Hospital admissions |
| Relating to the length of time and reasons for hospital admissions |
| Section 15: Current measurements |
| Relating to current height, weight, and head circumference measurements |
| Section 16: Day activities and school options |
| Relating to schooling and issues surrounding schooling |
| Section 17: Quality of life for individuals with MECP2 duplication syndrome |
| Relating to the quality of life of individuals with MDS |
|
| Part 2: Questions About the Caregiver and Family |
| Section 1: Family structure and demographics |
| Relating to family circumstances for those caring for a child or adult with MDS |
| Section 2: Family quality of life |
| Relating to quality of life of immediate family |
|
| Section 3: Caregiver quality of life |
| Relating to caregiver health and wellbeing |
|
| Characteristics | n/N (%) |
|---|---|
| Gender | |
| Male | 134/154 (87) |
| Female | 20/154 (13) |
| Age at census date (years) | |
| 0–<5 | 41/154 (27) |
| 5–<10 | 42/154 (27) |
| 10–<15 | 34/154 (22) |
| 15–<20 | 14/154 (9) |
| 20+ | 23/154 (15) |
| Mode of inheritance | |
| Inherited | 49/128 (38) |
| De novo | 21/128 (16) |
| Unconfirmed | 58/128 (45) |
| Age at genetic diagnosis (years) | |
| <1 | 34/143 (24) |
| 1–<2 | 34/143 (24) |
| 2–<5 | 29/143 (20) |
| 5–<15 | 31/143 (22) |
| 15–<30 | 12/143 (8) |
| 30+ | 2/143 (1) |
| Geographic distribution | |
| North America 1 | 76/154 (49) |
| Europe 2 | 51/154 (33) |
| Oceania 3 | 21/154 (14) |
| Asia 4 | 5/154 (3) |
| South America 5 | 1/154 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, D.; Downs, J.; Baynam, G.; Wilson, A.; Richmond, P.; Schmidt, A.; Decker, A.; Leonard, H. Development of an International Database for a Rare Genetic Disorder: The MECP2 Duplication Database (MDBase). Children 2022, 9, 1111. https://doi.org/10.3390/children9081111
Ta D, Downs J, Baynam G, Wilson A, Richmond P, Schmidt A, Decker A, Leonard H. Development of an International Database for a Rare Genetic Disorder: The MECP2 Duplication Database (MDBase). Children. 2022; 9(8):1111. https://doi.org/10.3390/children9081111
Chicago/Turabian StyleTa, Daniel, Jenny Downs, Gareth Baynam, Andrew Wilson, Peter Richmond, Aron Schmidt, Amelia Decker, and Helen Leonard. 2022. "Development of an International Database for a Rare Genetic Disorder: The MECP2 Duplication Database (MDBase)" Children 9, no. 8: 1111. https://doi.org/10.3390/children9081111
APA StyleTa, D., Downs, J., Baynam, G., Wilson, A., Richmond, P., Schmidt, A., Decker, A., & Leonard, H. (2022). Development of an International Database for a Rare Genetic Disorder: The MECP2 Duplication Database (MDBase). Children, 9(8), 1111. https://doi.org/10.3390/children9081111






