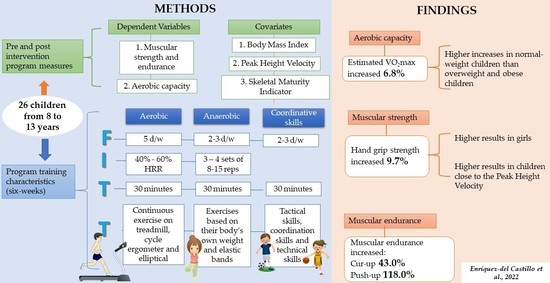
Strength and VO2max Changes by Exercise Training According to Maturation State in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Sample
2.3. Design
2.4. Physical Fitness Measurements
2.5. Body Mass Index
2.6. Biological Maturity Estimation
2.7. Training Program
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamah-Levy, T.; Cuevas-Nasu, L.; Gaona-Pineda, E.B.; Gómez-Acosta, L.M.; Morales-Ruán, M.; Hernández-Ávila, M.; Rivera-Dommarco, J.Á. Sobrepeso y obesidad en niños y adolescentes en México, actualización de la Encuesta Nacional de Salud y Nutrición de Medio Camino. Salud Publ. México 2018, 60, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, A.; Martin, A.; Janssen, X.; Wilson, M.G.; Gibson, A.M.; Hughes, A.; Reilly, J.J. Longitudinal changes in moderate-to-vigorous-intensity physical activity in children and adolescents: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e12953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Same World Health Organization (WHO). WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 12 September 2019).
- Eddolls, W.; McNarry, M.A.; Stratton, G.; Winn, C.; Mackintosh, K.A. High-Intensity Interval Training Interventions in Children and Adolescents: A Systematic Review. Sports Med. 2017, 47, 2363–2374. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Quan, M.; Zhuang, J. Effect of High-Intensity Interval Training versus Moderate-Intensity Continuous Training on Cardiorespiratory Fitness in Children and Adolescents: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2004; Volume 4. [Google Scholar]
- Cumming, S.P.; Sherar, L.B.; Esliger, D.W.; Riddoch, C.J.; Malina, R.M. Concurrent and prospective associations among biological maturation, and physical activity at 11 and 13 years of age. Scand. J. Med. Sci. Sports 2014, 24, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenowatz, C.; Wartha, O.; Klenk, J.; Brandstetter, S.; Wabitsch, M.; Steinacker, J. Differences in health behavior, physical fitness, and cardiovascular risk in early, average, and late mature children. Pediatr. Exerc. Sci. 2013, 25, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, S.J.; Ridgers, N.D. Relationships between maturity status, physical activity, and physical self-perceptions in primary school children. J. Sports Sci. J. Sport Sci. 2010, 28, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cumming, S.P.; Harrington, D.M.; Davis, M.J.; Edwardson, C.L.; Gorely, T.; Khunti, K.; Rowlands, A.V.; Yates, T.; Sherar, L.B. Maturational timing, physical self-perceptions and physical activity in UK adolescent females: Investigation of a mediated effects model. Ann. Hum. Biol. 2020, 47, 384–390. [Google Scholar]
- Ministry of Health of Mexico. General Law of Health in Research, Diario Oficial de la Federación Ultima reforma, México. 2014. Available online: https://www.gob.mx/conamer/prensa/reglamento-de-la-ley-general-de-salud-en-materia-de-investigacion-para-la-salud (accessed on 8 October 2021).
- Flores, L.A.; De León, L.G.; Ponce, P.J. Entrenamiento de la capacidad aerobia en prepúberes: Revisión sistemática. Rev. Andal. Med. Deport. 2019, 12, 121–127. [Google Scholar]
- Meredith, M.D.; Welk, G. (Eds.) Fitnessgram and Activitygram Test Administration Manual-Updated, 4th ed.; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Mahar, M.T.; Guerieri, A.M.; Hanna, M.S.; Kemble, C.D. Estimation of aerobic fitness from 20-m multistage shuttle run test performance. Am. J. Prev. Med. 2011, 41, 117–123. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T. Ridder Hd. In International Standards for Anthropometric Assessment. Lower Hutt; International Society for the Advancement of Kinanthropometry: Murcia, Spain, 2011. [Google Scholar]
- Esparza-Ros, F.; Vaquero-Cristóbal, R.; Marfell-Jones, M. Protocolo Internacional Para la Valoración Antropométrica. Perfil Completo; International Society for the Advancement of Kinanthropometry-ISAK: Murcia, Spain, 2019. [Google Scholar]
- Flores, L.A.; De León, L.G.; Fragoso, M.I. Skeletal age prediction model from percentage of adult height in children and adolescents. Sci. Rep. 2020, 10, 15768. [Google Scholar]
- Khamis, H.J.; Roche, A.F. Predicting adult stature without using skeletal age: The Khamis-Roche method. Pediatrics 1994, 94, 504–507. [Google Scholar] [PubMed]
- Malina, R.M.; Dompier, T.P.; Powell, J.W.; Barron, M.J.; Moor, M.T. Validation of a noninvasive maturity estimate relative to skeletal age in youth football players. Clin. J. Sport Med. 2007, 17, 362–368. [Google Scholar] [CrossRef]
- Mirwald, R.L.; Baxter-Jones, A.D.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar] [PubMed]
- Kröger, C.; Roth, K. Escuela de Balón: Guía Para Principiantes; Paidotribo: Barcelona, Spain, 2003. [Google Scholar]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 32, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Baquet, G.; van Praagh, E.; Berthoin, S. Endurance training and aerobic fitness in young people. Sports Med. 2003, 33, 1127–1143. [Google Scholar] [CrossRef] [Green Version]
- Özsu, İ. Effects of 6-Week Resistance Elastic Band Exercise on Functional Performances of 8–9 Year-Old Children. J. Educ. Train. Stud. 2018, 6, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Gastin, P.B.; Bennett, G. Late maturers at a performance disadvantage to their more mature peers in junior Australian football. J. Sports Sci. J. Sport Sci. 2014, 32, 563–571. [Google Scholar] [CrossRef]
- Till, K.; Cobley, S.O.; Hara, J.; Cooke, C.; Chapman, C. Considering maturation status and relative age in the longitudinal evaluation of junior rugby league players. Scand. J. Med. Sci. 2013, 24, 569–576. [Google Scholar] [CrossRef]
- Pullen, B.J.; Oliver, J.L.; Lloyd, R.S.; Knight, C.J. Relationships between Athletic Motor Skill Competencies and Maturity, Sex, Physical Performance, and Psychological Constructs in Boys and Girls. Children 2022, 9, 375. [Google Scholar] [CrossRef]
- Živković, M.; Stojiljković, N.; Trajković, N.; Stojanović, N.; Đošić, A.; Antić, V.; Stanković, N. Speed, Change of Direction Speed, and Lower Body Power in Young Athletes and Nonathletes According to Maturity Stage. Children 2022, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Albaladejo-Saura, M.; Vaquero-Cristóbal, R.; García-Roca, J.A.; Esparza-Ros, F. The Effect of Age, Biological Maturation and Birth Quartile in the Kinanthropometric and Physical Fitness Differences between Male and Female Adolescent Volleyball Players. Children 2022, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Albaladejo-Saura, M.; Vaquero-Cristóbal, R.; González-Gálvez, N.; Esparza-Ros, F. Relationship between Biological Maturation, Physical Fitness, and Kinanthropometric Variables of Young Athletes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 328. [Google Scholar] [CrossRef] [PubMed]
- Nevill, A.M.; Negra, Y.; Myers, T.D.; Duncan, M.J.; Chaabene, H.; Granacher, U. Are Early or Late Maturers Likely to be Fitter in the General Population? Int. J. Environ. Res. Public Health 2021, 18, 497. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Hoshikawa, S.; Hirose, N. Longitudinal Age-Related Morphological and Physiological Changes in Adolescent Male Basketball Players. J. Sci. Med. Sport 2019, 18, 751–757. [Google Scholar]
- Radnor, J.M.; Oliver, J.L.; Waugh, C.M.; Myer, G.D.; Lloyd, R.S. The influence of maturity status on muscle architecture in school-aged boys. Pediatr. Exerc. Sci. 2020, 32, 89–96. [Google Scholar] [CrossRef]
- Dobbs, I.J.; Oliver, J.L.; Wong, M.A.; Moore, I.S.; Lloyd, R.S. Effects of a 12-Week Training Program on Isometric and Dynamic Force-Time Characteristics in Pre–and Post–Peak Height Velocity Male Athletes. J. Strength. Cond. Res. 2020, 34, 653–662. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ranganathan, P. Study designs: Part 4–interventional studies. Perspect. Clin. Res. 2019, 10, 137–140. [Google Scholar] [CrossRef]
- Pastor, J.C.; Gil, P.; Tortosa, M.; Martínez, J. Efectos de un programa de actividad física extracurricular en niños de primer ciclo de ESO con sobrepeso y obesidad. Rev. Psicol. Deporte 2012, 21, 379–385. [Google Scholar]
| Variable | Girls (n = 15) | Boys (n = 11) | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Chronological age (years) | 11.0 ± 1.0 | 10.9 ± 0.9 | 0.838 |
| Skeletal age (years) | 11.1 ± 1.5 | 10.4 ± 1.3 | 0.148 |
| SMI (years) | 0.1 ± 1.0 | −0.6 ± 0.6 | 0.047 |
| BMI (kg/m2) | 19.5 ± 3.0 | 20.3 ± 5.6 | 0.919 |
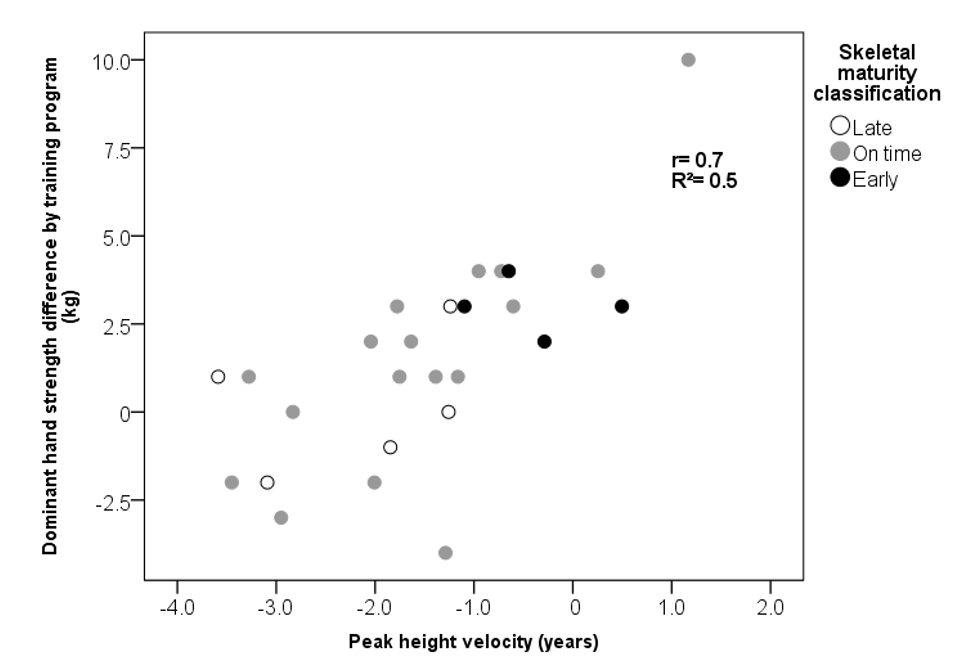
| PHV (years) | −0.8 ± 0.9 | −2.4 ± 1.0 | 0.001 |
| APHV (years) | 11.9 ± 0.4 | 13.3 ± 0.3 | 0.001 |
| VO2max (mL*kg*min) | 43.5 ± 3.1 | 47.6 ± 5.5 | 0.022 |
| PACER (laps) | 17.7 ± 5.5 | 20.4 ± 9.5 | 0.489 |
| Trunk-lift (repetitions) | 24.9 ± 4.5 | 18.4 ± 4.4 | 0.001 |
| Push-up (repetitions) | 6.0 ± 5.1 | 8.5 ± 10.1 | 0.919 |
| Curl-up (repetitions) | 10.9 ± 9.4 | 5.6 ± 7.1 | 0.281 |
| Hand grip strength D (kg) | 15.8 ± 4.8 | 15.1 ± 5.7 | 0.646 |
| Hand grip strength ND (kg) | 14.8 ± 4.2 | 14.5 ± 6.1 | 0.574 |
| Variable | PRE | POST | p Value | Effect Size |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Chronological age (years) | 11.0 ± 0.9 | 11.1 ± 0.9 | 0.001 | 0.11 |
| SA (years) | 10.8 ± 1.4 | 10.9 ± 1.4 | 0.001 | 0.07 |
| SMI (years) | −0.2 ± 0.9 | −0.2 ± 0.9 | 0.008 | 0.01 |
| BMI (kg/m2) | 19.8 ± 4.2 | 19.8 ± 4.0 | 0.378 | 0.01 |
| VO2max | 45.2 ± 4.7 | 48.3 ± 5.7 | 0.001 | 0.60 |
| PACER (laps) | 18.8 ± 7.4 | 27.9 ± 11.7 | 0.001 | 0.95 |
| Trunk-lift (repetitions) | 22.1 ± 5.5 | 22.4 ± 6.9 | 0.797 | 0.04 |
| Push-up (repetitions) | 7.0 ± 7.6 | 15.3 ± 8.8 | 0.001 | 1.02 |
| Curl-up (repetitions) | 8.6 ± 8.8 | 12.3 ± 9.6 | 0.034 | 0.40 |
| Hand grip strength D (kg) | 15.5 ± 5.1 | 17.0 ± 6.2 | 0.016 | 0.26 |
| Hand grip strength ND (kg) | 14.6 ± 4.9 | 15.8 ± 5.3 | 0.024 | 0.23 |
| Sources of Variation | Sum of Squares Type III | Fd | F | p Value | Partial eta Squared | Observed Power |
|---|---|---|---|---|---|---|
| VO2max (R2 = 0.3) | ||||||
| BMI basal | 27.2 | 1 | 6.6 | 0.017 | 0.22 | 0.69 |
| Sex | 4.0 | 1 | 1.0 | 0.333 | 0.04 | 0.16 |
| Error | 95.4 | 23 | ||||
| Total | 375.8 | 26 | ||||
| Dominant Hand Grip Strength (R2 = 0.2) | ||||||
| Sex | 46.0 | 1 | 6.8 | 0.016 | 0.22 | 0.71 |
| Error | 162.5 | 24 | ||||
| Total | 264.0 | 26 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enríquez-del-Castillo, L.A.; Ornelas-López, A.; De León, L.G.; Cervantes-Hernández, N.; Quintana-Mendias, E.; Flores, L.A. Strength and VO2max Changes by Exercise Training According to Maturation State in Children. Children 2022, 9, 938. https://doi.org/10.3390/children9070938
Enríquez-del-Castillo LA, Ornelas-López A, De León LG, Cervantes-Hernández N, Quintana-Mendias E, Flores LA. Strength and VO2max Changes by Exercise Training According to Maturation State in Children. Children. 2022; 9(7):938. https://doi.org/10.3390/children9070938
Chicago/Turabian StyleEnríquez-del-Castillo, Liliana Aracely, Andrea Ornelas-López, Lidia G. De León, Natanael Cervantes-Hernández, Estefanía Quintana-Mendias, and Luis Alberto Flores. 2022. "Strength and VO2max Changes by Exercise Training According to Maturation State in Children" Children 9, no. 7: 938. https://doi.org/10.3390/children9070938
APA StyleEnríquez-del-Castillo, L. A., Ornelas-López, A., De León, L. G., Cervantes-Hernández, N., Quintana-Mendias, E., & Flores, L. A. (2022). Strength and VO2max Changes by Exercise Training According to Maturation State in Children. Children, 9(7), 938. https://doi.org/10.3390/children9070938