The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
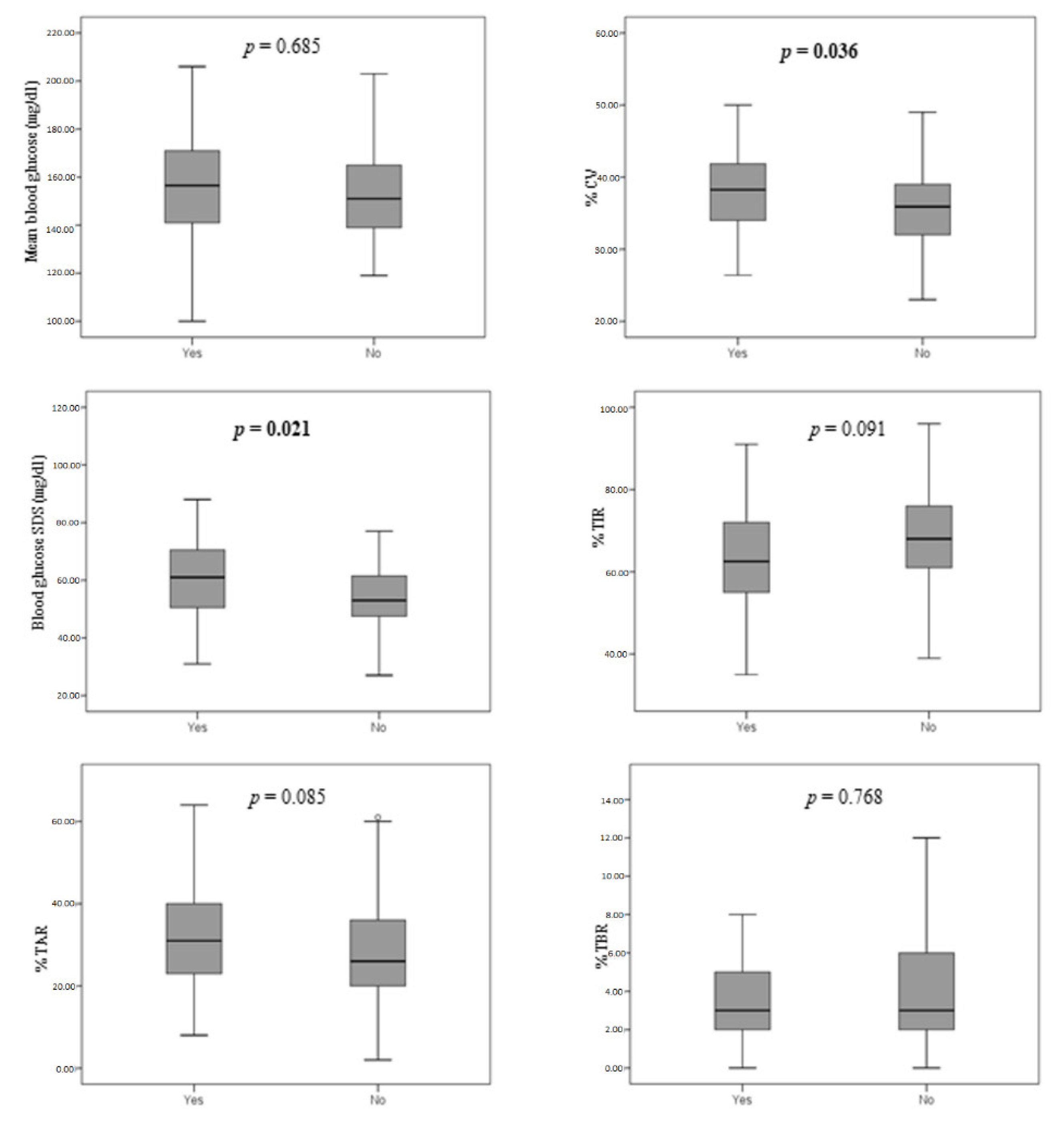
3. Results
3.1. Associated Factors
3.2. Glycemic Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- Cefalu, W.T.; Rosenstock, J.; LeRoith, D.; Riddle, M.C. Insulin’s Role in Diabetes Management: After 90 Years, Still Considered the Essential Black Dress. Diabetes Care 2015, 38, 2200–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrito, L.; Passanisi, S.; Bonfanti, R.; Cherubini, V.; Minuto, N.; Schiaffini, R.; Scaramuzza, A. Efficacy of advanced hybrid closed loop systems for the management of type 1 diabetes in children. Minerva Pediatr. 2021, 73, 474–485. [Google Scholar] [CrossRef]
- Lombardo, F.; Salzano, G.; Crisafulli, G.; Panasiti, I.; Alibrandi, A.; Messina, M.F.; Pajno, G.B.; Caminiti, L.; Passanisi, S. Allergic contact dermatitis in pediatric patients with type 1 diabetes: An emerging issue. Diabetes Res. Clin. Pract. 2020, 162, 108089. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Stockamp, B.; Haastert, B. Prevalence of lipohypertrophy in insulin-treated diabetic patients and predisposing factors. Exp. Clin. Endocrinol. Diabetes 1996, 104, 106–110. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Liu, C.; Chen, Y. A meta-analysis and meta-regression on the prevalence of lipohypertrophy in diabetic patients on insulin therapy. Therapies 2021, 76, 617–628. [Google Scholar] [CrossRef]
- Heinemann, L. Insulin absorption from lipodystrophic areas: A (neglected) source of trouble for insulin therapy? J. Diabetes Sci. Technol. 2010, 4, 750–753. [Google Scholar] [CrossRef] [Green Version]
- Fujikura, J.; Fujimoto, M.; Yasue, S.; Noguchi, M.; Masuzaki, H.; Hosoda, K.; Tachibana, T.; Sugihara, H.; Nakao, K. Insulin-induced lipohypertrophy: Report of a case with histopathology. Endocr. J. 2005, 52, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Renold, A.E.; Marble, A.; Fawcett, D.W. Action of insulin on deposition of glycogen and storage of fat in adipose tissue. Endocrinology 1950, 46, 55–66. [Google Scholar] [CrossRef]
- Babiker, A.; Datta, V. Lipoatrophy with insulin analogues in type I diabetes. Arch. Dis. Child. 2011, 96, 101–102. [Google Scholar] [CrossRef] [Green Version]
- Raile, K.; Noelle, V.; Landgraf, R.; Schwarz, H.P. Insulin antibodies are associated with lipoatrophy but also with lipohypertrophy in children and adolescents with type 1 diabetes. Exp. Clin. Endocrinol. Diabetes 2001, 109, 393–396. [Google Scholar] [CrossRef]
- Salgin, B.; Meissner, T.; Beyer, P.; Haberland, H.; Borkenstein, M.; Fussenegger, J.; Brand, U.; Hauffa, B.P.; Hungele, A.; Holl, R.W. Lipoatrophy is associated with an increased risk of Hashimoto’s thyroiditis and coeliac disease in female patients with type 1 diabetes. Horm. Res. Paediatr. 2013, 79, 368–372. [Google Scholar] [CrossRef]
- Lopez, X.; Castells, M.; Ricker, A.; Velazquez, E.F.; Mun, E.; Goldfine, A.B. Human insulin analog—Induced lipoatrophy. Diabetes Care 2008, 31, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Young, R.J.; Hannan, W.J.; Frier, B.M.; Steel, J.M.; Duncan, L.J. Diabetic lipohypertrophy delays insulin absorption. Diabetes Care 1984, 7, 479–480. [Google Scholar] [CrossRef]
- Famulla, S.; Hövelmann, U.; Fischer, A.; Coester, H.V.; Hermanski, L.; Kaltheuner, M.; Kaltheuner, L.; Heinemann, L.; Heise, T.; Hirsch, L. Insulin Injection into Lipohypertrophic Tissue: Blunted and More Variable Insulin Absorption and Action and Impaired Postprandial Glucose Control. Diabetes Care 2016, 39, 1486–1492. [Google Scholar] [CrossRef] [Green Version]
- Kordonouri, O.; Lauterborn, R.; Deiss, D. Lipohypertrophy in young patients with type 1 diabetes. Diabetes Care 2002, 25, 634. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, T.A.; Escudier, V. Poor glycaemic control caused by insulin induced lipohypertrophy. BMJ 2003, 327, 383–384. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, H.B.; Hougaard, P.; Swift, P.; Hansen, L.; Holl, R.W.; Hoey, H.; Bjoerndalen, H.; De Beaufort, C.; Chiarelli, F.; Danne, T.; et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009, 32, 1384–1390. [Google Scholar] [CrossRef] [Green Version]
- Gentile, S.; Guarino, G.; Giancaterini, A.; Guida, P.; Strollo, F.; AMD-OSDI Italian Injection Technique Study Group. A suitable palpation technique allows to identify skin lipohypertrophic lesions in insulin-treated people with diabetes. SpringerPlus 2016, 5, 563. [Google Scholar] [CrossRef] [Green Version]
- Deeb, A.; Abdelrahman, L.; Tomy, M.; Suliman, S.; Akle, M.; Smith, M.; Strauss, K. Impact of Insulin Injection and Infusion Routines on Lipohypertrophy and Glycemic Control in Children and Adults with Diabetes. Diabetes Ther. 2019, 10, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, M.A.; El-Kafoury, A.A.; El-Araby, R.I. Lipohypertrophy in children and adolescents with type 1 diabetes and the associated factors. BMC Res. Notes 2011, 4, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsadik, A.G.; Atey, T.M.; Nedi, T.; Fantahun, B.; Feyissa, M. Effect of Insulin-Induced Lipodystrophy on Glycemic Control among Children and Adolescents with Diabetes in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. J. Diabetes Res. 2018, 2018, 4910962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, N.; Zhang, X.; Zhao, F.; Wang, Y.; He, H. Prevalence of lipohypertrophy in insulin-treated diabetes patients: A systematic review and meta-analysis. J. Diabetes Investig. 2018, 9, 536–543. [Google Scholar] [CrossRef]
- Frid, A.H.; Hirsch, L.J.; Menchior, A.R.; Morel, D.R.; Strauss, K.W. Worldwide Injection Technique Questionnaire Study: Injecting Complications and the Role of the Professional. Mayo Clin. Proc. 2016, 91, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Conwell, L.S.; Pope, E.; Artiles, A.M.; Mohanta, A.; Daneman, A.; Daneman, D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J. Pediatr. 2008, 152, 622–628. [Google Scholar] [CrossRef]
- Passanisi, S.; Salzano, G.; Lombardo, F. Skin involvement in paediatric patients with type 1 diabetes. Curr. Diabetes Rev. 2022, 18, e030921196145. [Google Scholar] [CrossRef]
- Richardson, T.; Kerr, D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am. J. Clin. Dermatol. 2003, 4, 661–667. [Google Scholar] [CrossRef]
- Radermecker, R.P.; Piérard, G.E.; Scheen, A.J. Lipodystrophy reactions to insulin: Effects of continuous insulin infusion and new insulin analogs. Am. J. Clin. Dermatol. 2007, 8, 21–28. [Google Scholar] [CrossRef]
- Kordonouri, O.; Biester, T.; Schnell, K.; Hartmann, R.; Tsioli, C.; Fath, M.; Datz, N.; Danne, T. Lipoatrophy in children with type 1 diabetes: An increasing incidence? J. Diabetes Sci. Technol. 2015, 9, 206–208. [Google Scholar] [CrossRef] [Green Version]
- Rosenbloom, A.L. Insulin injection lipoatrophy recidivus. Pediatr. Diabetes 2014, 15, 73–74. [Google Scholar] [CrossRef]
- Pozzuoli, G.M.; Laudato, M.; Barone, M.; Crisci, F.; Pozzuoli, B. Errors in insulin treatment management and risk of lipohypertrophy. Acta Diabetol. 2018, 55, 67–73. [Google Scholar] [CrossRef]
- Blanco, M.; Hernández, M.T.; Strauss, K.W.; Amaya, M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013, 39, 445–453. [Google Scholar] [CrossRef]
- Danne, T.; Phillip, M.; Buckingham, B.A.; Jarosz-Chobot, P.; Saboo, B.; Urakami, T.; Battelino, T.; Hanas, R.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Insulin treatment in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19 (Suppl. S27), 115–135. [Google Scholar] [CrossRef]
- Al Hayek, A.A.; Robert, A.A.; Braham, R.B.; Al Dawish, M.A. Frequency of Lipohypertrophy and Associated Risk Factors in Young Patients with Type 1 Diabetes: A Cross-Sectional Study. Diabetes Ther. 2016, 7, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Demir, G.; Er, E.; Atik Aktınok, Y.; Özen, S.; Darcan, Ş.; Gökşen, D. Local complications of insulin administration sites and effect on diabetes management. J. Clin. Nurs. 2021, in press. [Google Scholar] [CrossRef]
- Johansson, U.B.; Amsberg, S.; Hannerz, L.; Wredling, R.; Adamson, U.; Arnqvist, H.J.; Lins, P.E. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care 2005, 28, 2025–2027. [Google Scholar] [CrossRef] [Green Version]
- De Coninck, C.; Frid, A.; Gaspar, R.; Hicks, D.; Hirsch, L.; Kreugel, G.; Liersch, J.; Letondeur, C.; Sauvanet, J.P.; Tubiana, N.; et al. Results and analysis of the 2008–2009 Insulin Injection Technique Questionnaire survey. J. Diabetes 2010, 2, 168–179. [Google Scholar] [CrossRef]
- Piona, C.; Ventrici, C.; Marcovecchio, L.; Chiarelli, F.; Maffeis, C.; Bonfanti, R.; Rabbone, I. Long-term complications of type 1 diabetes: What do we know and what do we need to understand? Minerva Pediatr. 2021, 73, 503–522. [Google Scholar] [CrossRef]
- Šoupal, J.; Škrha Jr, J.; Fajmon, M.; Horová, E.; Mráz, M.; Škrha, J.; Prázný, M. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol. Ther. 2014, 16, 198–203. [Google Scholar] [CrossRef]
| Variables | Percentage and Mean ± SDS | Median (IQR) |
|---|---|---|
| Age (years) | 11.9 ± 4.7 | 12.9 (9.1; 15.7) |
| Gender Male Female | 123 (58%) 89 (42%) | |
| Duration of diabetes (years) | 4.8 ± 3.4 | 4 (2; 7) |
| BMI Z-score | 0.72 ± 0.96 | 0.78 (0.18; 1.39) |
| Insulin treatment type Multiple daily injections Insulin pump | 92 (43.4%) 120 (56.6%) | |
| Daily insulin dose (IU/kg/die) | 0.84 ± 0.26 | 0.82 (0.70; 1.00) |
| Last year mean value HbA1c (%) | 6.8 ± 1.6 | 7.0 (6.5; 7.5) |
| Last year mean value HbA1c (mmol/mol) | 53.9 ± 9.8 | 53 (49; 58) |
| Glucose monitoring system Self-monitoring of blood glucose Flash or continuous glucose monitoring | 61 (28.8%) 151 (71.2%) | |
| Presence of lipodystrophy Lipohypertrophy Single Multiple LipoatrophyNone | 94 (44.3%) 50 (23.6%) 44 (20.7%) 2 (0.9%) 116 (54.7%) | |
| Awareness of the problem Yes No | 191 (90.5%) 20 (9.5%) |
| Lipodystrophy | No Lipodystrophy | p-Value | |
|---|---|---|---|
| Number of patients | 96 | 116 | |
| Gender Male Female | 64 (66.7%) 32 (33.3%) | 59 (50.9%) 57 (49.1%) | 0.020 |
| Age (years) | 11.8 ± 4.8 | 12.1 ± 4.6 | 0.673 |
| Duration of diabetes (years) | 5.2 ± 3.6 | 4.5 ± 3.2 | 0.215 |
| BMI z-score | 0.62 ± 0.94 | 0.80 ± 0.96 | 0.165 |
| Insulin treatment type Multiple daily injections Insulin pump | 47 (49.0%) 49 (51.0%) | 45 (38.8%) 71 (61.2%) | 0.137 |
| Rotation of injection/insertion sites Yes No | 77 (80.2%) 19 (19.8%) | 104 (89.7%) 12 (10.3%) | 0.050 |
| Awareness of the problem Yes No | 81 (84.4%) 15 (15.6%) | 110 (95.7%) 5 (4.3%) | 0.005 |
| Creams application Yes No | 35 (36.5%) 61 (63.5%) | 34 (29.3%) 82 (70.7%) | 0.269 |
| Daily insulin dose (IU/kg/die) | 0.88 ± 0.25 | 0.81 ± 0.27 | 0.085 |
| Last year mean value HbA1c (%) | 6.8 ± 1.7 | 6.9 ± 1.5 | 0.397 |
| Last year mean value HbA1c (mmol/mol) | 53.8 ± 9.3 | 53.9 ± 10.3 |
| Variables | B | 95% CI | p-Value |
|---|---|---|---|
| Lipodystrophy | 2.050 | 0.062–4.161 | 0.027 |
| Lipodystrophy | 2.113 | 0.026-4.199 | 0.047 |
| Age | −0.226 | −0.441-0.012 | 0.039 |
| Lipodystrophy | 2.208 | 0.118-4.297 | 0.039 |
| Age | −0.225 | −0.439-0.011 | 0.039 |
| Gender | 1.262 | −0.812–3.337 | 0.231 |
| Lipodystrophy | 1.959 | −0.164–4.083 | 0.070 |
| Age | −0.206 | −0.424–0.011 | 0.063 |
| BMI Z-score | 0.024 | −1.033–1.081 | 0.964 |
| Lipodystrophy | 1.953 | −0.157–4.063 | 0.069 |
| Age | −0.158 | −0.380–0.064 | 0.162 |
| BMI Z-score | −0.134 | −1.192–0.924 | 0.803 |
| Gender | 1.455 | −0.649–3.560 | 0.174 |
| Type of therapy | −2.229 | −4.694–0.235 | 0.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, F.; Bombaci, B.; Alibrandi, A.; Visalli, G.; Salzano, G.; Passanisi, S. The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes. Children 2022, 9, 1087. https://doi.org/10.3390/children9071087
Lombardo F, Bombaci B, Alibrandi A, Visalli G, Salzano G, Passanisi S. The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes. Children. 2022; 9(7):1087. https://doi.org/10.3390/children9071087
Chicago/Turabian StyleLombardo, Fortunato, Bruno Bombaci, Angela Alibrandi, Giulia Visalli, Giuseppina Salzano, and Stefano Passanisi. 2022. "The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes" Children 9, no. 7: 1087. https://doi.org/10.3390/children9071087
APA StyleLombardo, F., Bombaci, B., Alibrandi, A., Visalli, G., Salzano, G., & Passanisi, S. (2022). The Impact of Insulin-Induced Lipodystrophy on Glycemic Variability in Pediatric Patients with Type 1 Diabetes. Children, 9(7), 1087. https://doi.org/10.3390/children9071087









