Motor Influence in Developing Auditory Spatial Cognition in Hemiplegic Children with and without Visual Field Disorder
Abstract
:1. Introduction
2. Materials and Methods
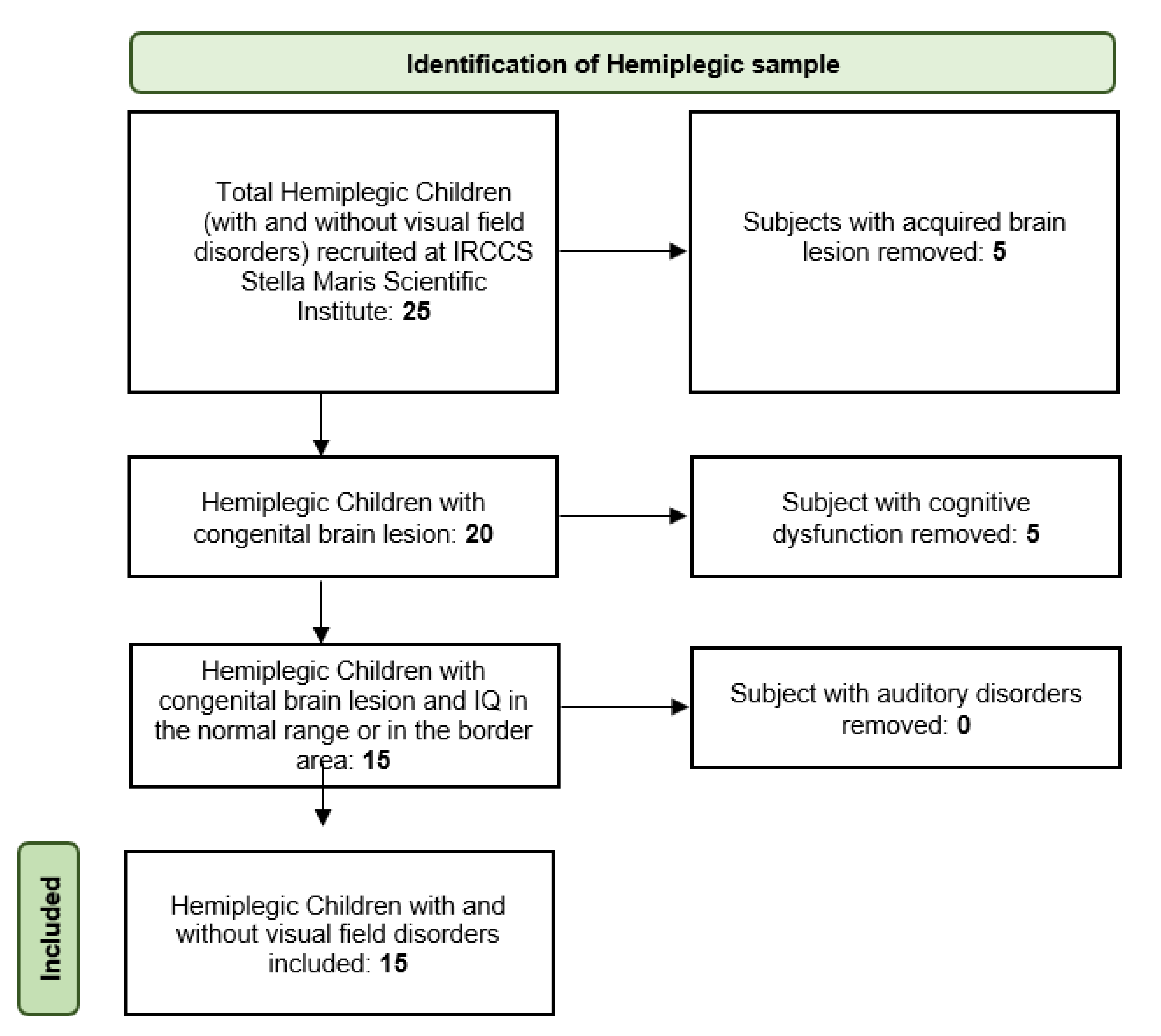
2.1. Patients
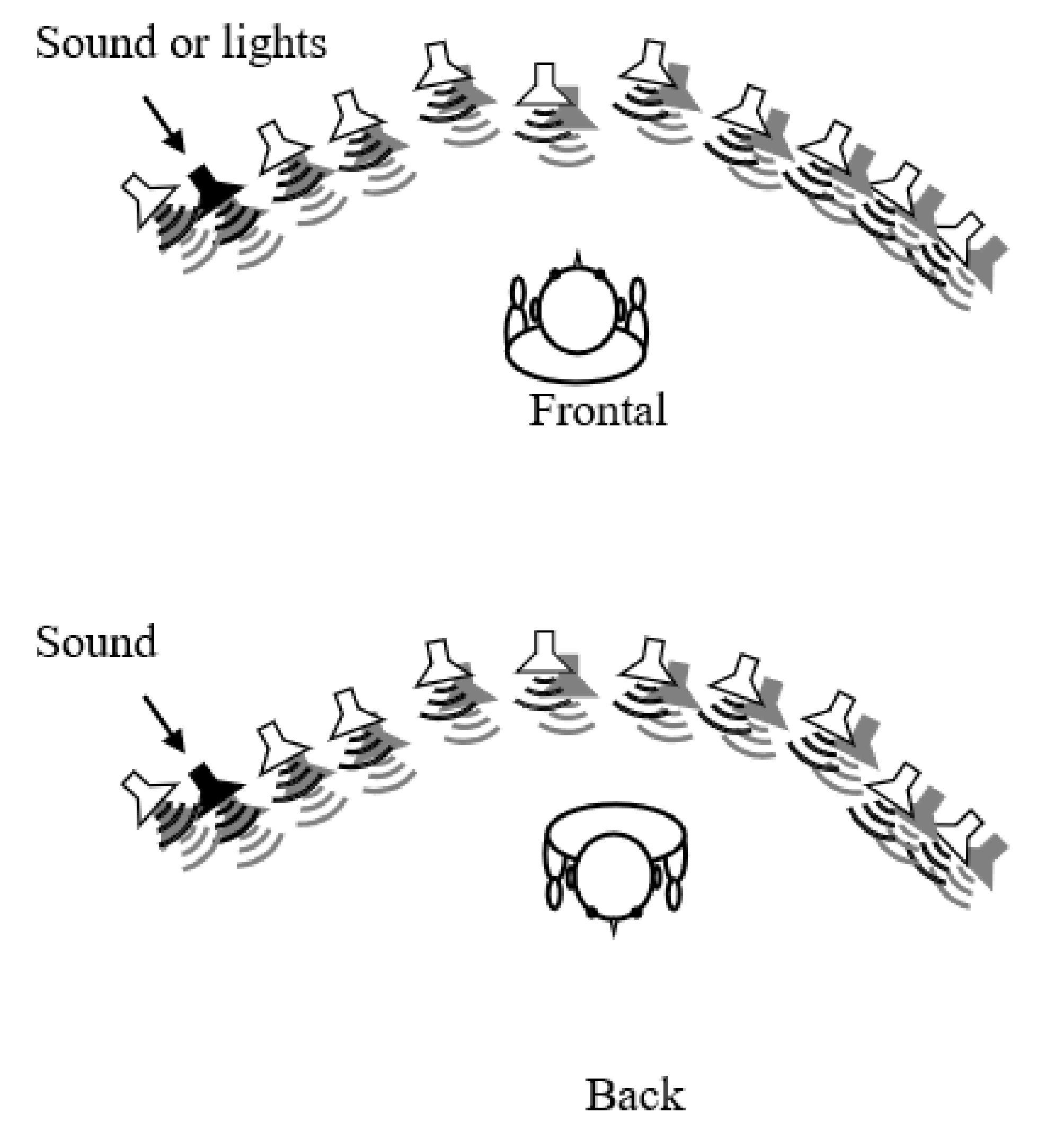
2.2. Setup and Stimuli
2.3. Tasks and Procedures
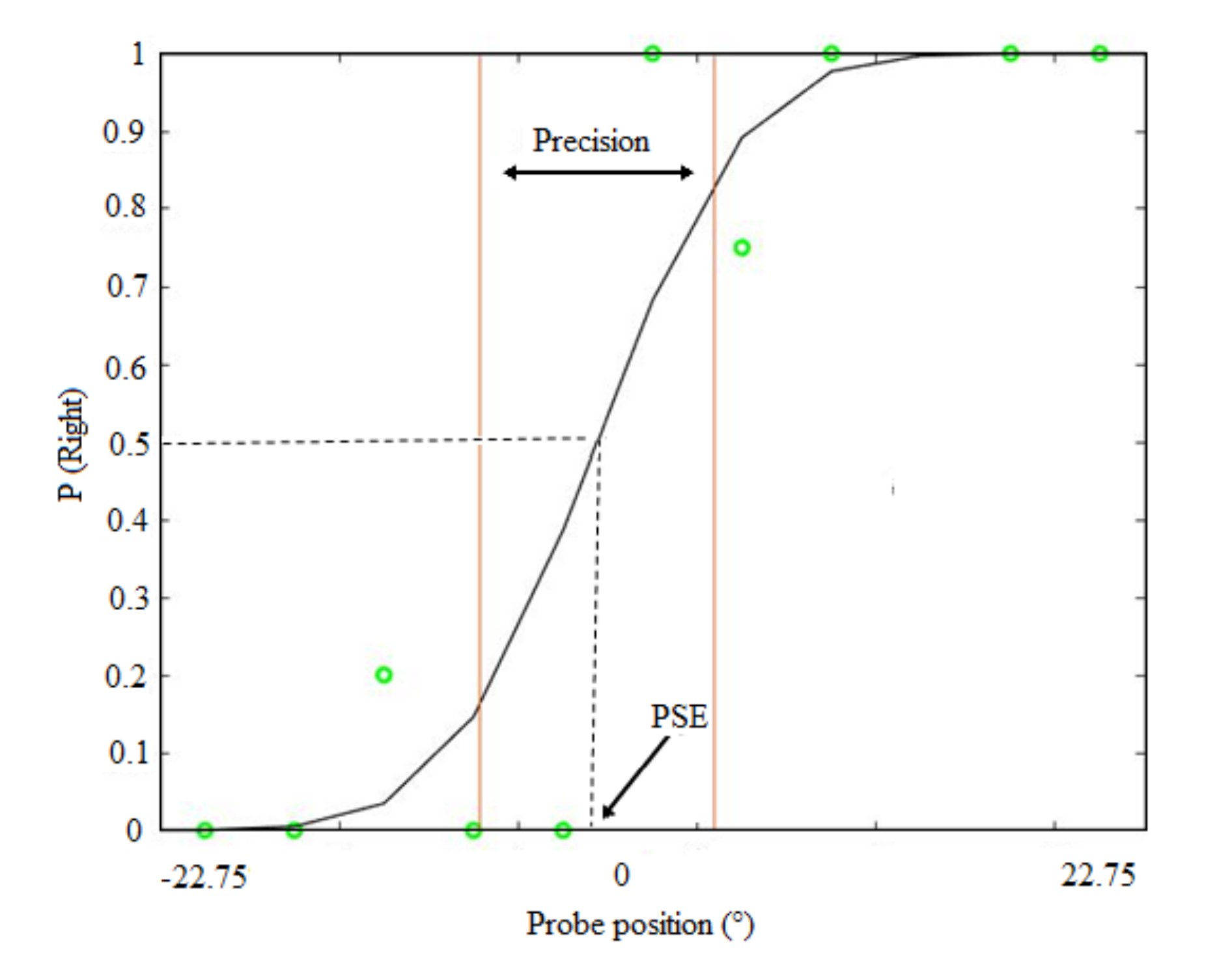
2.4. Analysis
3. Results
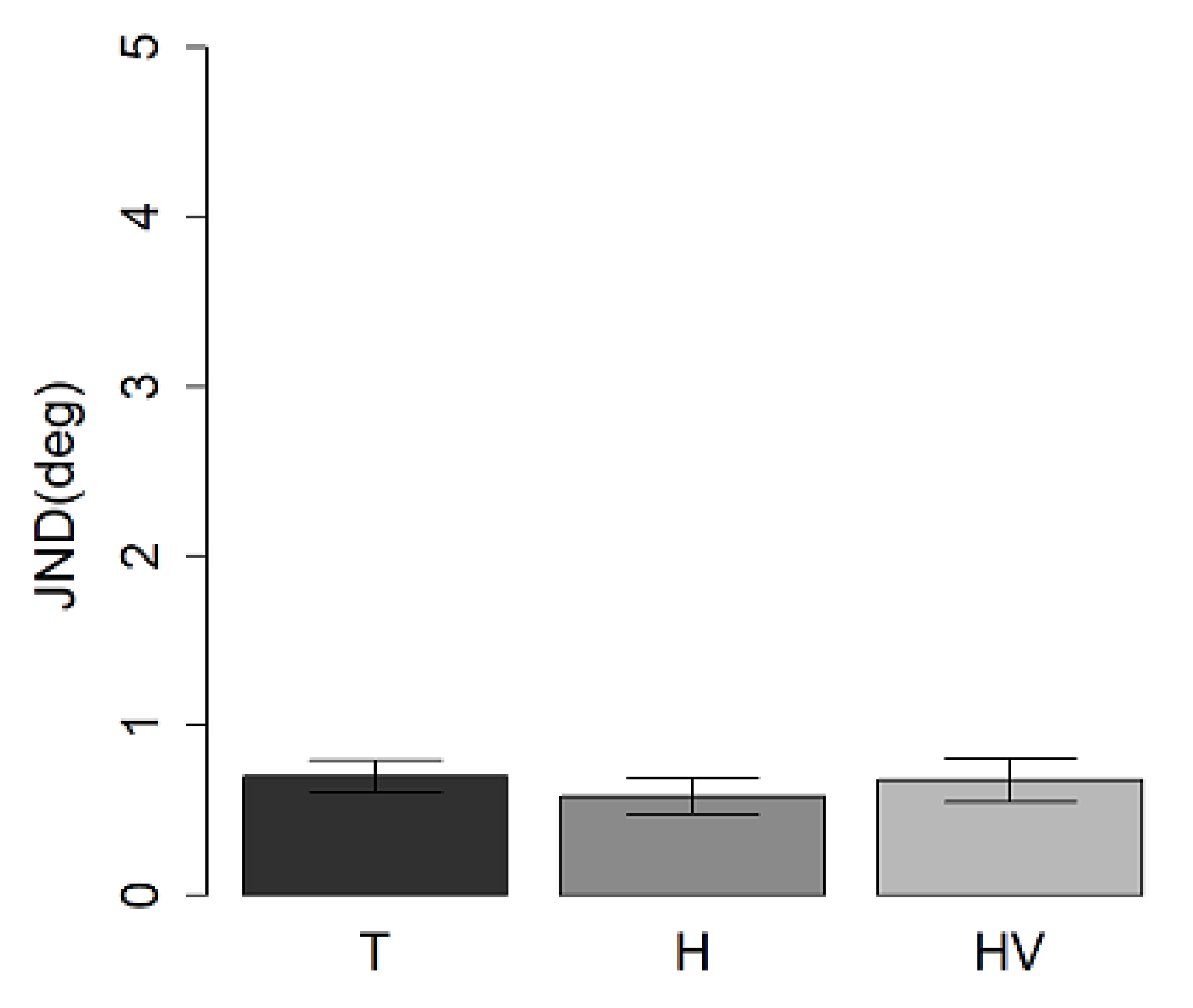
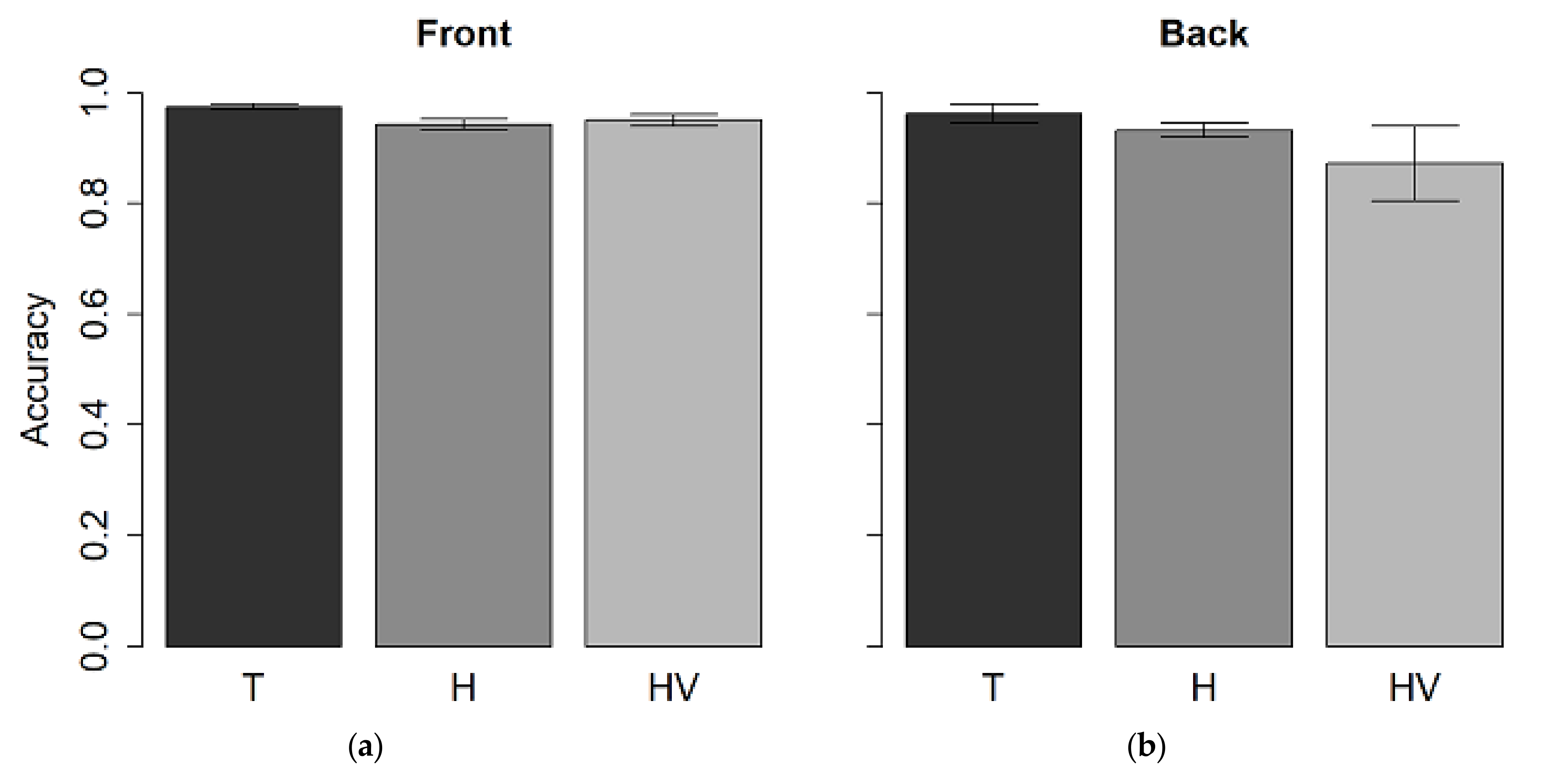
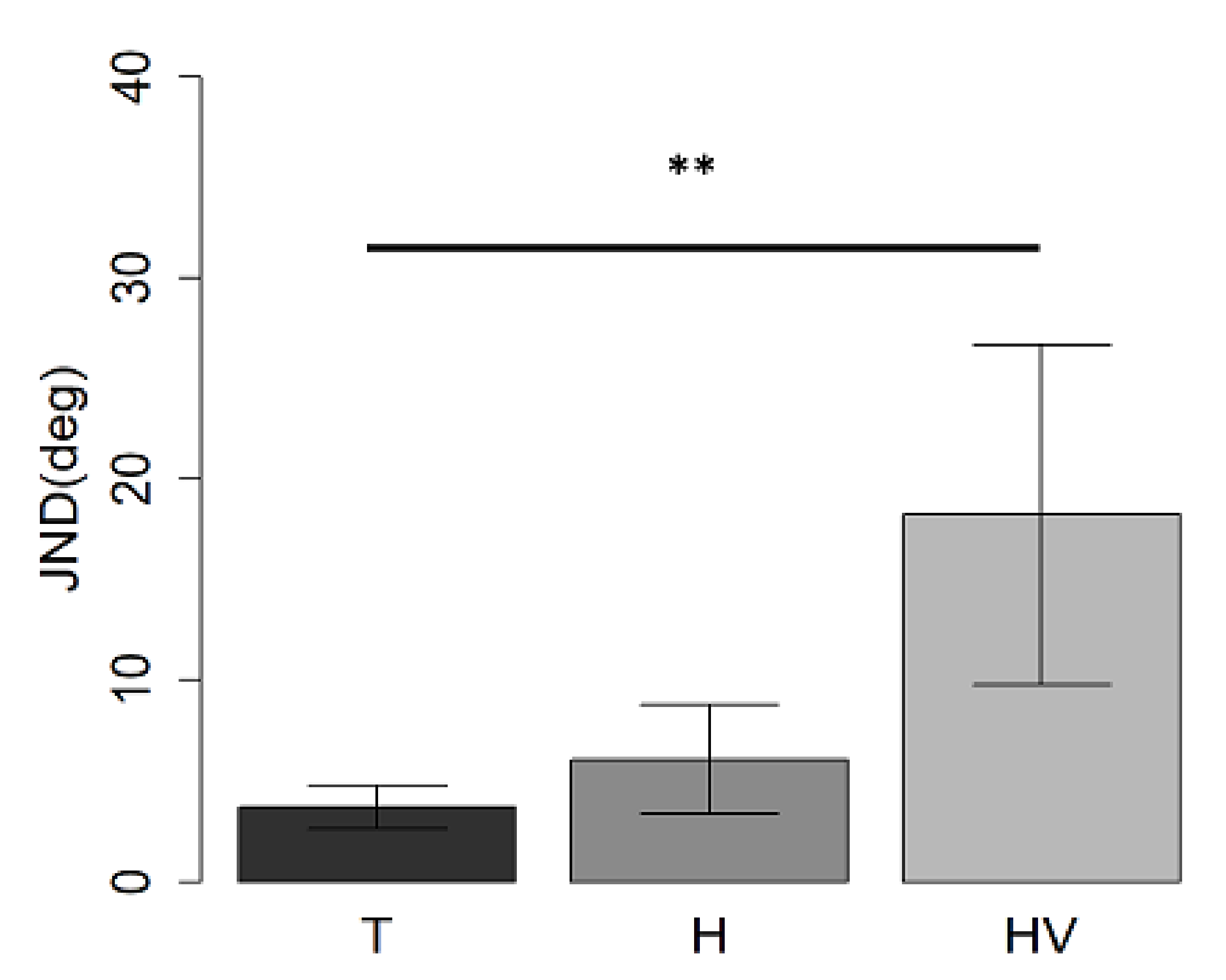
3.1. Auditory Spatial Discrimination Task
3.2. Pitch Discrimination Task
3.3. Visual-Spatial Discrimination
3.4. Correlation between MACS Values and JND at Front Auditory Spatial Discrimination Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roder, B.; Rosler, F.; Spence, C. Early vision impairs tactile perception in the blind. Curr. Biol. 2004, 14, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, M.; Lourenco, S.F. Development of spatial cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Sandini, G.; Martinoli, C.; Burr, D.C. Impairment of auditory spatial localization in congenitally blind human subjects. Brain 2014, 137, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallar, G.; Guariglia, C.; Nico, D.; Bisiach, E. Spatial hemineglect in back space. Brain 1995, 118, 467–472. [Google Scholar]
- Farne, A.; Ladavas, E. Auditory peripersonal space in humans. J. Cogn. Neurosci. 2002, 14, 1030–1043. [Google Scholar]
- Saj, A.; Vuilleumier, P. Neglect: Remembering the space left behind. Curr. Biol. 2007, 17, R1060–R1062. [Google Scholar] [CrossRef]
- Viaud-Delmon, I.; Brugger, P.; Landis, T. Hemineglect: Take a look at the back space. Ann. Neurol. 2007, 62, 418–422. [Google Scholar] [CrossRef]
- di Pellegrino, G.; Ladavas, E. Peripersonal space in the brain. Neuropsychologia 2015, 66, 126–133. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fadiga, L.; Fogassi, L.; Gallese, V. The space around us. Science 1997, 277, 190–191. [Google Scholar] [CrossRef]
- Aimola, L.; Schindler, I.; Simone, A.M.; Venneri, A. Near and far space neglect: Task sensitivity and anatomical substrates. Neuropsychologia 2012, 50, 1115–1123. [Google Scholar] [CrossRef]
- Clery, J.; Guipponi, O.; Wardak, C.; Ben Hamed, S. Neuronal bases of peripersonal and extrapersonal spaces, their plasticity and their dynamics: Knowns and unknowns. Neuropsychologia 2015, 70, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Noel, J.-P.; Galli, G.; Canzoneri, E.; Marmaroli, P.; Lissek, H.; Blanke, O. Body part-centered and full body-centered peripersonal space representations. Sci. Rep. 2015, 5, 18603. [Google Scholar] [CrossRef] [PubMed]
- Ladavas, E.; Serino, A. Action-dependent plasticity in peripersonal space representations. Cogn. Neuropsychol. 2008, 25, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Zampini, M.; Torresan, D.; Spence, C.; Murray, M.M. Auditory-somatosensory multisensory interactions in front and rear space. Neuropsychologia 2007, 45, 1869–1877. [Google Scholar] [CrossRef]
- Occelli, V.; Spence, C.; Zampini, M. Audiotactile interactions in front and rear space. Neurosci. Biobehav. Rev. 2011, 35, 589–598. [Google Scholar] [CrossRef]
- Finocchietti, S.; Cappagli, G.; Gori, M. Encoding audio motion: Spatial impairment in early blind individuals. Front. Psychol. 2015, 6, 1357. [Google Scholar] [CrossRef]
- Bronkhorst, A.W. The cocktail-party problem revisited: Early processing and selection of multi-talker speech. Atten. Percept. Psychophys. 2015, 77, 1465–1487. [Google Scholar] [CrossRef] [Green Version]
- Kidd, G.; Arbogast, T.L.; Mason, C.R.; Gallun, F.J. The advantage of knowing where to listen. J. Acoust. Soc. Am. 2005, 118, 3415–3804. [Google Scholar] [CrossRef]
- Pasqualotto, A.; Proulx, M.J. The role of visual experience for the neural basis of spatial cognition. Neurosci. Biobehav. Rev. 2012, 36, 1179–1187. [Google Scholar] [CrossRef]
- Bremmer, F.; Klam, F.; Duhamel, J.-R.; Ben Hamed, S.; Graf, W. Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur. J. Neurosci. 2002, 16, 1569–1586. [Google Scholar] [CrossRef]
- Millar, S. Self-referent and movement cues in coding spatial location by blind and sighted children. Perception 1981, 10, 255–264. [Google Scholar] [PubMed]
- Paillard, J. Motor and representational framing of space. Oxf. Univ. Press Ed. 1991, 163–182. [Google Scholar]
- Berti, A.; Frassinetti, F. When far becomes near: Remapping of space by tool use. J. Cogn. Neurosci. 2000, 12, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Matelli, M.; Luppino, G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage 2001, 14, S27–S32. [Google Scholar] [PubMed] [Green Version]
- Aggius-Vella, E.; Claudio, C.; Finocchietti, S.; Gori, M. Audio Spatial Representation Around the Body. Front. Psychol. 2017, 8, 1932. [Google Scholar] [CrossRef]
- Canzoneri, E.; Ubaldi, S.; Rastelli, V.; Finisguerra, A.; Bassolino, M.; Serino, A. Tool-use reshapes the boundaries of body and peripersonal space representations. Exp. Brain Res. 2013, 228, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Aggius-Vella, E.; Claudio, C.; Finocchietti, S.; Gori, M. Audio Motor Training at the Foot Level Improves Space Representation. Front. Integr. Neurosci. 2017, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Cappagli, G.; Cocchi, E.; Gori, M. Auditory and proprioceptive spatial impairments in blind children and adults. Dev. Sci. 2017, 20, e12374. [Google Scholar] [CrossRef]
- Finocchietti, S.; Cappagli, G.; Gori, M. Auditory Spatial Recalibration in Congenital Blind Individuals. Front Neurosci. 2017, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Kopinska, A.; Harris, L.R. Spatial representation in body coordinates: Evidence from errors in remembering positions of visual and auditory targets after active eye, head, and body movements. Can. J. Exp. Psychol. 2003, 57, 23–37. [Google Scholar] [CrossRef]
- Vercillo, T.; Burr, D.; Gori, M. Early visual deprivation severely compromises the auditory sense of space in congenitally blind children. Dev. Psychol. 2016, 52, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, M.; Cappagli, G.; Tonelli, A.; Baud-Bovy, G.; Finocchietti, S. Devices for visually impaired people: High technological devices with low user acceptance and no adaptability for children. Neurosci Biobehav. Rev. 2016, 69, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konoike, N.; Kotozaki, Y.; Miyachi, S.; Miyauchi, C.M.; Yomogida, Y.; Akimoto, Y.; Kuraoka, K.; Sugiura, M.; Kawashima, R.; Nakamura, K. Rhythm information represented in the fronto-parieto-cerebellar motor system. Neuroimage 2012, 63, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hove, M.J.; Keller, P.E. Impaired movement timing in neurological disorders: Rehabilitation and treatment strategies. Ann. N. Y. Acad. Sci. 2015, 1337, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcolm, M.P.; Massie, C.; Thaut, M. Rhythmic auditory-motor entrainment improves hemiparetic arm kinematics during reaching movements: A pilot study. Top Stroke Rehabil. 2009, 16, 69–79. [Google Scholar] [CrossRef]
- McIntosh, G.C.; Brown, S.H.; Rice, R.R.; Thaut, M.H. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Thaut, M.H.; McIntosh, G.; Rice, R. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J. Neurol. Sci. 1997, 151, 207–212. [Google Scholar] [CrossRef]
- Thaut, M.H.; McIntosh, G.C.; Rice, R.R.; Miller, R.A.; Rathbun, J.; Brault, J.M. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 1996, 11, 193–200. [Google Scholar] [CrossRef]
- Martin, K.; Trauner, D.A. Auditory neglect in children following perinatal stroke. Behav. Brain Res. 2019, 359, 878–885. [Google Scholar]
- Fazzi, E.; Signorini, S.G.; Bomba, M.; Luparia, A.; Lanners, J.; Balottin, U. Reach on sound: A key to object permanence in visually impaired children. Early Hum. Dev. 2011, 87, 289–296. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant Development: Third Edition. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011. [Google Scholar]
- Cappagli, G.; Finocchietti, S.; Baud-Bovy, G.; Cocchi, E.; Gori, M. Multisensory Rehabilitation Training Improves Spatial Perception in Totally but Not Partially Visually Deprived Children. Front. Integr. Neurosci. 2017, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, B.; Spelke, E.; Gleitman, H. Gleitman, Spatial knowledge in a young blind child. Cognition 1984, 16, 225–260. [Google Scholar] [CrossRef]
- Aggius-Vella, E.; Claudio, C.; Kolarik, A.J.; Gori, M. The Role of Visual Experience in Auditory Space Perception around the Legs. Sci. Rep. 2019, 9, 10992. [Google Scholar] [CrossRef] [PubMed]


| Lesion | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient ID | Sex | Age (Years) | Side | Site | Type | Hemiplegia | Visual Field | MACS Scale |
| 1 | f | 8 | R | T,P | PVL:> involvement of the right side | L | L HH | 3 |
| 2 | m | 11 | L | F,T,P | MCA Infarction: Main branch | R | R HH | 5 |
| 3 | m | 7 | L | sc | Venous Infarction | R | No | 2 |
| 4 | f | 8 | L | P,sc | Parasagittal arterial infarction | R | No | 1 |
| 5 | f | 11 | R | sc | MCA: lenticular infarction | L | L Reduction | 4 |
| 6 | f | 7 | L | T,P,O | MCA Infarction: Main branch | R | R HH | 3 |
| 7 | f | 8 | R | P,sc | PVL | L | No | 1 |
| 8 | f | 8 | R | sc | Venous infarction | L | No | 1 |
| 9 | m | 10 | L | F,T, P,sc | MCA Infarction: Main branch | R | No | 1 |
| 10 | m | 5 | R | F | Parasagittal arterial infarction | L | No | 2 |
| 11 | f | 5 | L | F,P,sc | MCA Infarction: Main branch | R | No | 3 |
| 12 | m | 11 | L | sc | MCA: lenticular infarction | R | No | 3 |
| 13 | f | 14 | L | F,T,P,sc | MCA Infarction: Main branch | R | RQ upper | 2 |
| 14 | f | 7 | L | F,T,P,O,sc | MCA Infarction: Main branch | R | R HH | 3 |
| 15 | m | 14 | L | sc | MCA: lenticular infarction | R | No | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggius-Vella, E.; Gori, M.; Campus, C.; Petri, S.; Tinelli, F. Motor Influence in Developing Auditory Spatial Cognition in Hemiplegic Children with and without Visual Field Disorder. Children 2022, 9, 1055. https://doi.org/10.3390/children9071055
Aggius-Vella E, Gori M, Campus C, Petri S, Tinelli F. Motor Influence in Developing Auditory Spatial Cognition in Hemiplegic Children with and without Visual Field Disorder. Children. 2022; 9(7):1055. https://doi.org/10.3390/children9071055
Chicago/Turabian StyleAggius-Vella, Elena, Monica Gori, Claudio Campus, Stefania Petri, and Francesca Tinelli. 2022. "Motor Influence in Developing Auditory Spatial Cognition in Hemiplegic Children with and without Visual Field Disorder" Children 9, no. 7: 1055. https://doi.org/10.3390/children9071055
APA StyleAggius-Vella, E., Gori, M., Campus, C., Petri, S., & Tinelli, F. (2022). Motor Influence in Developing Auditory Spatial Cognition in Hemiplegic Children with and without Visual Field Disorder. Children, 9(7), 1055. https://doi.org/10.3390/children9071055






