Spontaneous Pneumomediastinum in Children with Viral Infections: Report of Three Cases Related to Rhinovirus or Respiratory Syncytial Virus Infection
Abstract
:1. Introduction
2. Case Reports
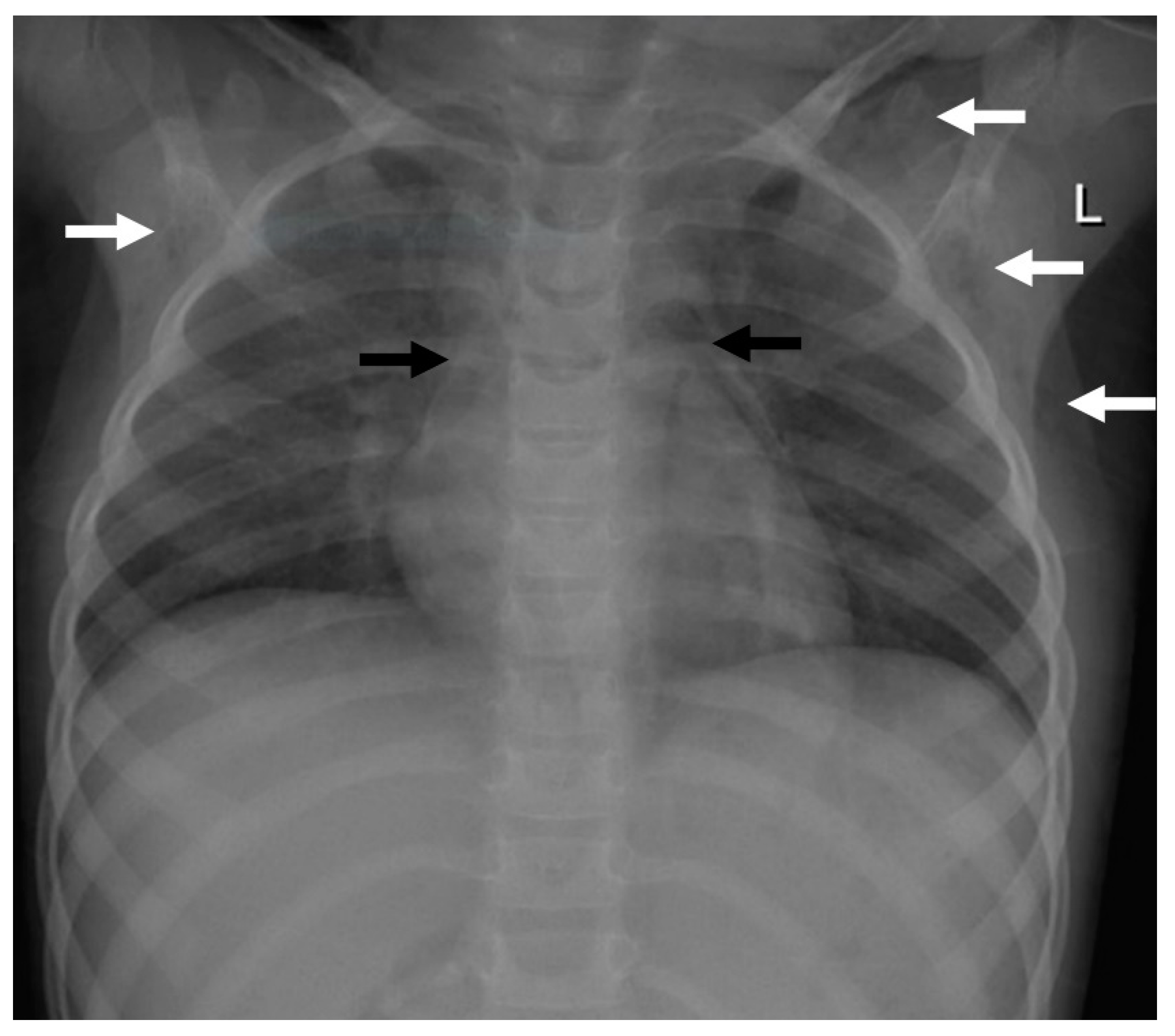
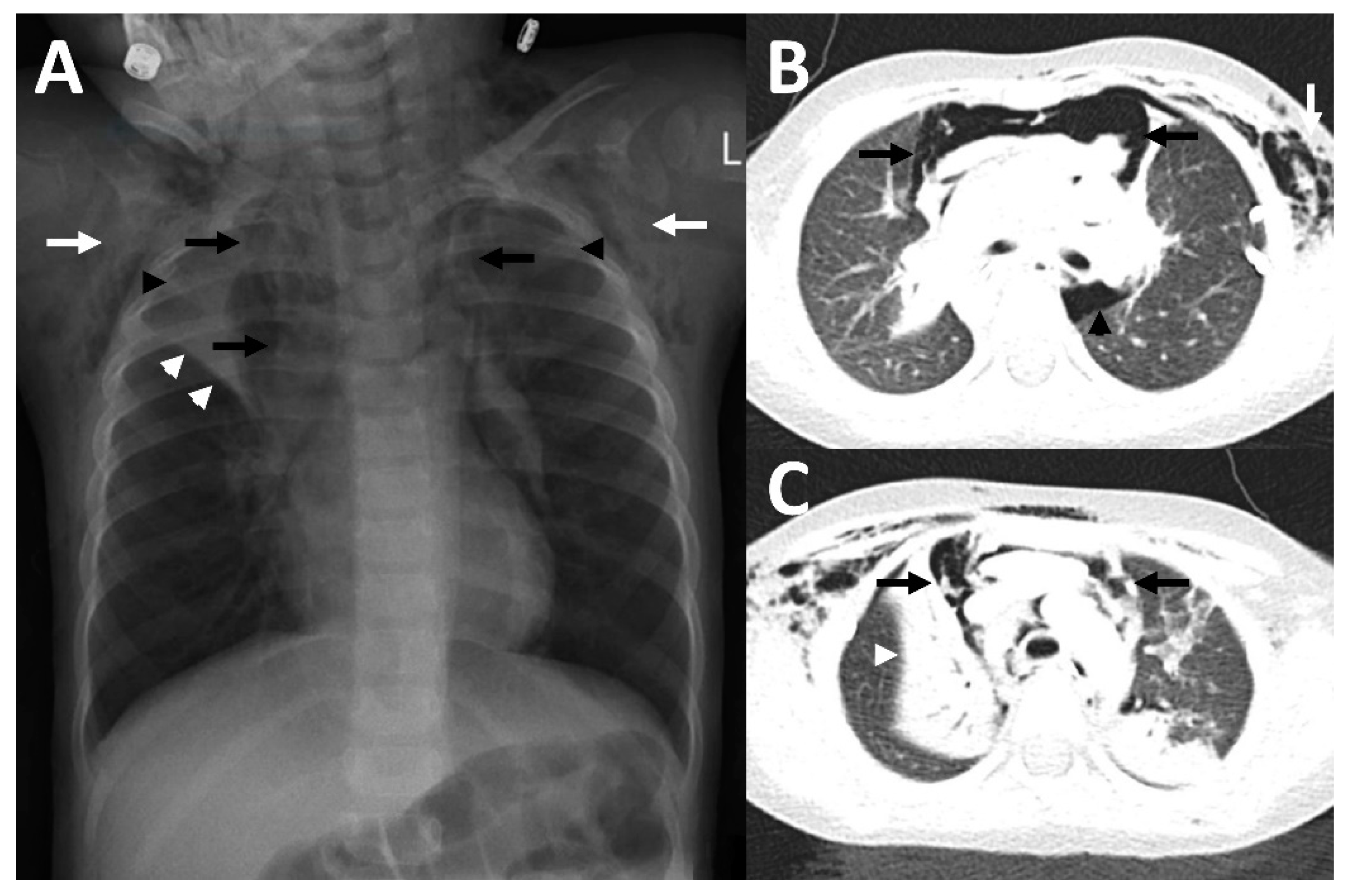
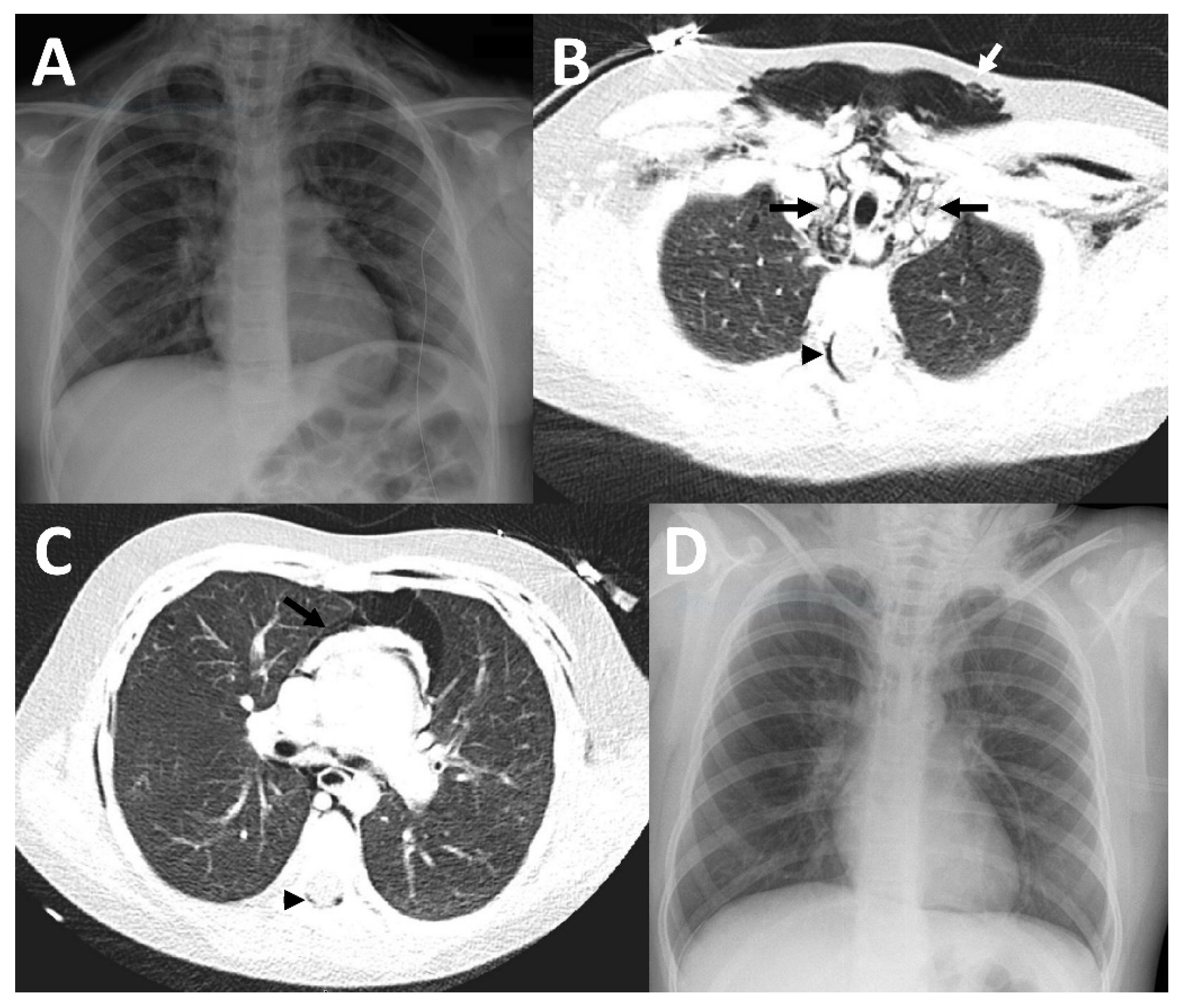
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bullaro, F.M.; Bartoletti, S.C. Spontaneous pneumomediastinum in children: A literature review. Pediatr. Emerg. Care 2007, 23, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Verma, S.; Grullon, J.; Esquire, A.; Patel, P.; Talwar, A. Spontaneous pneumomediastinum: Time for consensus. N. Am. J. Med. Sci. 2013, 5, 460–464. [Google Scholar] [PubMed]
- Chalumeau, M.; Le Clainche, L.; Sayeg, N.; Sannier, N.; Michel, J.L.; Marianowski, R.; Jouvet, P.; Scheinmann, P.; de Blic, J. Spontaneous pneumomediastinum in children. Pediatr. Pulmonol. 2001, 31, 67–75. [Google Scholar] [CrossRef]
- Fitzwater, J.W.; Silva, N.N.; Knight, C.G.; Malvezzi, L.; Ramos-Irizarry, C.; Burnweit, C.A. Management of spontaneous pneumomediastinum in children. J. Pediatr. Surg. 2015, 50, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, K.A.; Williams, A.E.; Langham, J.J.W.; Wu, L.; Krafty, R.T.; Furtado, A.D.; Zuckerbraun, N.S.; Manole, M.D. Management and Outcomes of Spontaneous Pneumomediastinum in Children. Pediatr. Emerg. Care 2019, 37, e1051–e1056. [Google Scholar] [CrossRef] [PubMed]
- Iuorio, A.; Nagar, F.; Attianese, L.; Grasso, A.; Torretta, G.; Fusco, P.; Ferrara, M.; Ferraro, F. Spontaneous Pneumomediastinum and Pneumothorax in Nonintubated COVID-19 Patients: A Multicenter Case Series. Am. J. Case Rep. 2021, 22, e933405. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef] [PubMed]
- Jagosz, M.; Guzik, W.; Moczala, L.; Rydel, M.; Misiolek, H.; Bialka, S. Young convalescent COVID-19 pneumonia with extensive pneumomediastinum emphysema: Case report. Clin. Case Rep. 2022, 10, e05543. [Google Scholar] [CrossRef] [PubMed]
- Emiralioglu, N.; Ozcan, H.N.; Oguz, B.; Yalcin, E.; Dogru, D.; Ozcelik, U.; Kiper, N. Pneumomediastinum, pneumorrhachis and subcutaneous emphysema associated with viral infections: Report of three cases. Pediatr. Int. 2015, 57, 1038–1040. [Google Scholar] [CrossRef]
- Dehmel, M.; Demirakca, S.; Jordan, A.; Dahlheim, M.; Diehm, T.; Schroten, H.; Tenenbaum, T. Pneumomediastinum and subcutaneus emphysema—An uncommon presentation of respiratory-syncytial virus infection in an infant. Klin Padiatr. 2013, 225, 230–231. [Google Scholar] [CrossRef]
- Manoha, C.; Espinosa, S.; Aho, S.L.; Huet, F.; Pothier, P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J. Clin. Virol. 2007, 38, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Oertel, M.F.; Korinth, M.C.; Reinges, M.H.; Krings, T.; Terbeck, S.; Gilsbach, J.M. Pathogenesis, diagnosis and management of pneumorrhachis. Eur. Spine J. 2006, 15 (Suppl. 5), 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caceres, M.; Ali, S.Z.; Braud, R.; Weiman, D.; Garrett, H.E., Jr. Spontaneous pneumomediastinum: A comparative study and review of the literature. Ann. Thorac. Surg. 2008, 86, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Abbas, P.I.; Akinkuotu, A.C.; Peterson, M.L.; Mazziotti, M.V. Spontaneous pneumomediastinum in the pediatric patient. Am. J. Surg. 2015, 210, 1031–1035, discussion 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.R.; Pellaton, R.; Rochat, C.P. Pediatric Spontaneous Pneumomediastinum: Narrative Literature Review. Pediatr. Emerg. Care 2017, 33, 370–374. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leinert, J.L.; Perez Ortiz, A.; Rafat, N. Spontaneous Pneumomediastinum in Children with Viral Infections: Report of Three Cases Related to Rhinovirus or Respiratory Syncytial Virus Infection. Children 2022, 9, 1040. https://doi.org/10.3390/children9071040
Leinert JL, Perez Ortiz A, Rafat N. Spontaneous Pneumomediastinum in Children with Viral Infections: Report of Three Cases Related to Rhinovirus or Respiratory Syncytial Virus Infection. Children. 2022; 9(7):1040. https://doi.org/10.3390/children9071040
Chicago/Turabian StyleLeinert, Johanna L., Alba Perez Ortiz, and Neysan Rafat. 2022. "Spontaneous Pneumomediastinum in Children with Viral Infections: Report of Three Cases Related to Rhinovirus or Respiratory Syncytial Virus Infection" Children 9, no. 7: 1040. https://doi.org/10.3390/children9071040
APA StyleLeinert, J. L., Perez Ortiz, A., & Rafat, N. (2022). Spontaneous Pneumomediastinum in Children with Viral Infections: Report of Three Cases Related to Rhinovirus or Respiratory Syncytial Virus Infection. Children, 9(7), 1040. https://doi.org/10.3390/children9071040





