Nutrition in Pediatric Intensive Care: A Narrative Review
Abstract
:1. Introduction
2. Nutritional Status Assessment
3. Nutritional Support Initiation
4. Optimal Energy and Protein Delivery for a Critically Ill Child
5. Macronutrients
5.1. Proteins
5.2. Carbohydrates
5.3. Lipids
6. Fluid, Electrolytes
7. Micronutrients
8. Enteral Nutrition
9. Parenteral Nutrition
10. Examples of Enteral and Parenteral Feeding Prescriptions in PICU
- Nutrition screening and anthropometric measurement—(e.g., Strongkids):
- Low risk of malnutrition, weight = 25 kg, height 120 cm.
- Indirect calorimetry or Schofield equation E and protein target calculation:
- E target: 1061 kcal/d, protein target at least: 37.5 g/d.
- Gastric tube insertion, and after initial stabilization (fluid resuscitation, antibiotics), gastric nutrition started according to local protocol within 24 h from admission:
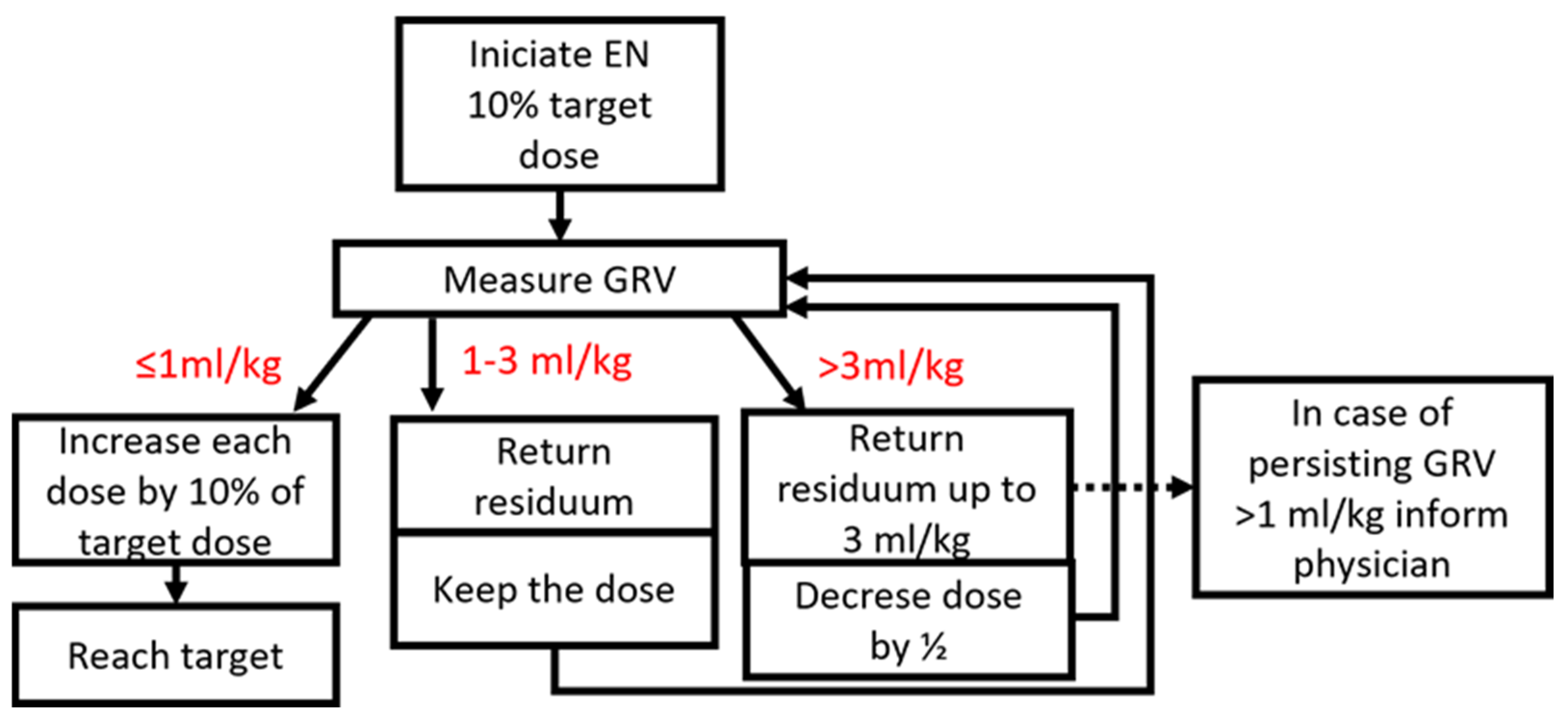
- Isocaloric enteral nutrition formula (1 kcal/1 mL, target 1061 mL/d), bolus form 5 × times per day—initial bolus 10% of estimated dose = 1061/5 times per day/10% ≈ 20 mL of initial dose, 40 mL of second dose, 60 mL of third dose, in the case of intolerance (GRV > 3 mL/kg, dose reduction and optimize to tolerance).
- Nutrition screening and anthropometric measurement—(e.g., Strongkids):
- High risk of malnutrition, weight = 35 kg, height 140 cm.
- Indirect calorimetry or Schofield equation E and protein target calculation:
- E target: 1276 kcal/d, protein target at least: 52.5 g/d.
- Gastric tube in situ, severe abdominal distension, enteral nutrition according to the surgeon currently contraindicated:
- Total parenteral nutrition (with added micronutrients) via central/peripherally inserted central line with frequently reassessment of the possibility of “trophic” enteral feeding.
- If individual PN—53 g of 10% amino acids (525 mL) + 36 g of 20% lipid emulsion (180 mL) + 178 g of 40% glucose (445 mL) + 40 mL of 10% NaCl + 35 mL of 7.5% KCl + 5 mL MgSO4 10% + 5 mL of Ca gluconicum + multivitamin + trace elements + 640 mL of aqua pro injectione (Holiday and Segar formula fluids 1875 mL/day).
11. Nutritional Status Evaluation during PICU Stay
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tume, L.N.; Valla, F.V.; Joosten, K.; Jotterand Chaparro, C.; Latten, L.; Marino, L.V.; Macleod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional Support for Children during Critical Illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) Metabolism, Endocrine and Nutrition Section Position Statement and Clinical Recommendations. Intensive Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosten, K.F.M.; Kerklaan, D.; Verbruggen, S.C.A.T. Nutritional Support and the Role of the Stress Response in Critically Ill Children. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Joosten, K.F.M.; Eveleens, R.D.; Verbruggen, S.C.A.T. Nutritional Support in the Recovery Phase of Critically Ill Children. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Puffelen, E.; Hulst, J.M.; Vanhorebeek, I.; Dulfer, K.; Van den Berghe, G.; Verbruggen, S.C.A.T.; Joosten, K.F.M. Outcomes of Delaying Parenteral Nutrition for 1 Week vs. Initiation Within 24 Hours Among Undernourished Children in Pediatric Intensive Care: A Subanalysis of the PEPaNIC Randomized Clinical Trial. JAMA Netw. Open 2018, 1, e182668. [Google Scholar] [CrossRef]
- Prince, N.J.; Brown, K.L.; Mebrahtu, T.F.; Parslow, R.C.; Peters, M.J. Weight-for-Age Distribution and Case-Mix Adjusted Outcomes of 14,307 Paediatric Intensive Care Admissions. Intensive Care Med. 2014, 40, 1132–1139. [Google Scholar] [CrossRef]
- Van Puffelen, E.; Hulst, J.M.; Vanhorebeek, I.; Dulfer, K.; Van den Berghe, G.; Joosten, K.F.M.; Verbruggen, S.C.A.T. Effect of Late versus Early Initiation of Parenteral Nutrition on Weight Deterioration during PICU Stay: Secondary Analysis of the PEPaNIC Randomised Controlled Trial. Clin. Nutr. 2020, 39, 104–109. [Google Scholar] [CrossRef]
- Mihatsch, W.; Fewtrell, M.; Goulet, O.; Molgaard, C.; Picaud, J.-C.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Calcium, Phosphorus and Magnesium. Clin. Nutr. 2018, 37, 2360–2365. [Google Scholar] [CrossRef] [Green Version]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Bechard, L.J.; Duggan, C.; Touger-Decker, R.; Parrott, J.S.; Rothpletz-Puglia, P.; Byham-Gray, L.; Heyland, D.; Mehta, N.M. Nutritional Status Based on Body Mass Index Is Associated with Morbidity and Mortality in Mechanically Ventilated Critically Ill Children in the PICU. Crit. Care Med. 2016, 44, 1530–1537. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.M.; Bechard, L.J.; Cahill, N.; Wang, M.; Day, A.; Duggan, C.P.; Heyland, D.K. Nutritional Practices and Their Relationship to Clinical Outcomes in Critically Ill Children—An International Multicenter Cohort Study. Crit. Care Med. 2012, 40, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Bechard, L.J.; Zurakowski, D.; Duggan, C.P.; Heyland, D.K. Adequate Enteral Protein Intake Is Inversely Associated with 60-d Mortality in Critically Ill Children: A Multicenter, Prospective, Cohort Study. Am. J. Clin. Nutr. 2015, 102, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, P.A.; Newth, C.J.L.; Leung, D.; Wetzel, R.C.; Khemani, R.G. Obesity and Mortality Risk in Critically Ill Children. Pediatrics 2016, 137, e20152035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza Menezes, F.; Leite, H.P.; Koch Nogueira, P.C. Malnutrition as an Independent Predictor of Clinical Outcome in Critically Ill Children. Nutr. Burbank 2012, 28, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.F.; Okay, T.S.; Leone, C.; Nichols, B.; Del Negro, G.M.; Vaz, F.A.C. Hospital Malnutrition and Inflammatory Response in Critically Ill Children and Adolescents Admitted to a Tertiary Intensive Care Unit. Clinics 2008, 63, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Briassoulis, G.; Zavras, N.; Hatzis, T. Malnutrition, Nutritional Indices, and Early Enteral Feeding in Critically Ill Children. Nutrition 2001, 17, 548–557. [Google Scholar] [CrossRef]
- Hulst, J.; Joosten, K.; Zimmermann, L.; Hop, W.; van Buuren, S.; Büller, H.; Tibboel, D.; van Goudoever, J. Malnutrition in Critically Ill Children: From Admission to 6 Months after Discharge. Clin. Nutr. 2004, 23, 223–232. [Google Scholar] [CrossRef]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. Pediatr. Crit. Care Med. 2017, 18, 675–715. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying Critically Ill Patients Who Benefit the Most from Nutrition Therapy: The Development and Initial Validation of a Novel Risk Assessment Tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef] [Green Version]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional Risk Screening (NRS 2002): A New Method Based on an Analysis of Controlled Clinical Trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Poisson-Salomon, A.S.; Colomb, V.; Brusset, M.C.; Mosser, F.; Berrier, F.; Ricour, C. Simple Pediatric Nutritional Risk Score to Identify Children at Risk of Malnutrition. Am. J. Clin. Nutr. 2000, 72, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Macleod, I.; Maclean, A.; Buchanan, E.; McGrogan, P.; Swinbank, I.; McAuley, M.; Wright, C.M.; Flynn, D.M. Performance of the Novel Paediatric Yorkhill Malnutrition Score (PYMS) in Hospital Practice. Clin. Nutr. 2011, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.; Dixon, M.; Crabtree, I.; Eaton-Evans, M.J.; McNulty, H. The Development and Evaluation of the Screening Tool for the Assessment of Malnutrition in Paediatrics (STAMP©) for Use by Healthcare Staff. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2012, 25, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.; Lawson, K.; Ramsey, R.; Dennis, N.; Hutchinson, Z.; Soh, X.Y.; Matsuyama, M.; Doolan, A.; Todd, A.; Elliott, A.; et al. Simple Nutrition Screening Tool for Pediatric Inpatients. JPEN J. Parenter. Enteral Nutr. 2016, 40, 392–398. [Google Scholar] [CrossRef]
- Chourdakis, M.; Hecht, C.; Gerasimidis, K.; Joosten, K.F.; Karagiozoglou-Lampoudi, T.; Koetse, H.A.; Ksiazyk, J.; Lazea, C.; Shamir, R.; Szajewska, H.; et al. Malnutrition Risk in Hospitalized Children: Use of 3 Screening Tools in a Large European Population. Am. J. Clin. Nutr. 2016, 103, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Wischmeyer, P.E.; San-Millan, I. Winning the War against ICU-Acquired Weakness: New Innovations in Nutrition and Exercise Physiology. Crit. Care 2015, 19 (Suppl. 3), S6. [Google Scholar] [CrossRef] [Green Version]
- Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.J.; Twisk, J.W.R.; Oudemans-van Straaten, H.M.; Weijs, P.J.M. Skeletal Muscle Quality as Assessed by CT-Derived Skeletal Muscle Density Is Associated with 6-Month Mortality in Mechanically Ventilated Critically Ill Patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [Green Version]
- Thibault, R.; Makhlouf, A.-M.; Mulliez, A.; Cristina Gonzalez, M.; Kekstas, G.; Kozjek, N.R.; Preiser, J.-C.; Rozalen, I.C.; Dadet, S.; Krznaric, Z.; et al. Fat-Free Mass at Admission Predicts 28-Day Mortality in Intensive Care Unit Patients: The International Prospective Observational Study Phase Angle Project. Intensive Care Med. 2016, 42, 1445–1453. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early Enteral Nutrition in Critically Ill Patients: ESICM Clinical Practice Guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Kreymann, K.G.; Berger, M.M.; Deutz, N.E.P.; Hiesmayr, M.; Jolliet, P.; Kazandjiev, G.; Nitenberg, G.; van den Berghe, G.; Wernerman, J.; DGEM (German Society for Nutritional Medicine); et al. ESPEN Guidelines on Enteral Nutrition: Intensive Care. Clin. Nutr. 2006, 25, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Singer, P.; Berger, M.M.; Van den Berghe, G.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C.; et al. ESPEN Guidelines on Parenteral Nutrition: Intensive Care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerklaan, D.; Augustus, M.E.; Hulst, J.M.; van Rosmalen, J.; Verbruggen, S.C.A.T.; Joosten, K.F.M. Validation of Ventilator-Derived VCO2 Measurements to Determine Energy Expenditure in Ventilated Critically Ill Children. Clin. Nutr. 2017, 36, 452–457. [Google Scholar] [CrossRef] [PubMed]
- van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; Sainz de Pipaon, M.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Amino Acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fivez, T.; Kerklaan, D.; Mesotten, D.; Verbruggen, S.; Wouters, P.J.; Vanhorebeek, I.; Debaveye, Y.; Vlasselaers, D.; Desmet, L.; Casaer, M.P.; et al. Early versus Late Parenteral Nutrition in Critically Ill Children. N. Engl. J. Med. 2016, 374, 1111–1122. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, E.; Vanhorebeek, I.; Joosten, K.F.M.; Wouters, P.J.; Van den Berghe, G.; Verbruggen, S.C.A.T. Early versus Late Parenteral Nutrition in Critically Ill, Term Neonates: A Preplanned Secondary Subgroup Analysis of the PEPaNIC Multicentre, Randomised Controlled Trial. Lancet Child Adolesc. Health 2018, 2, 505–515. [Google Scholar] [CrossRef]
- Mesotten, D.; Joosten, K.; van Kempen, A.; Verbruggen, S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Carbohydrates. Clin. Nutr. 2018, 37, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- NICE-SUGAR Study Investigators; Finfer, S.; Chittock, D.R.; Su, S.Y.-S.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef] [Green Version]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef]
- Jochum, F.; Moltu, S.J.; Senterre, T.; Nomayo, A.; Goulet, O.; Iacobelli, S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Fluid and Electrolytes. Clin. Nutr. 2018, 37, 2344–2353. [Google Scholar] [CrossRef] [Green Version]
- Chesney, C.R. The Maintenance Need for Water in Parenteral Fluid Therapy, by Malcolm A. Holliday, MD, and William E. Segar, MD, Pediatrics, 1957;19:823-832. Pediatrics 1998, 102, 229–230. [Google Scholar]
- Mahmoodpoor, A.; Shadvar, K.; Sanaie, S.; Hadipoor, M.R.; Pourmoghaddam, M.A.; Saghaleini, S.H. Effect of Vitamin C on Mortality of Critically Ill Patients with Severe Pneumonia in Intensive Care Unit: A Preliminary Study. BMC Infect. Dis. 2021, 21, 616. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Bronsky, J.; Campoy, C.; Braegger, C.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domellöf, M.; Szitanyi, P.; Simchowitz, V.; Franz, A.; Mimouni, F.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Iron and Trace Minerals. Clin. Nutr. 2018, 37, 2354–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichimaru, S. Methods of Enteral Nutrition Administration in Critically Ill Patients: Continuous, Cyclic, Intermittent, and Bolus Feeding. Nutr. Clin. Pract. 2018, 33, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Kolaček, S.; Puntis, J.W.L.; Hojsak, I.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Venous Access. Clin. Nutr. 2018, 37, 2379–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riskin, A.; Picaud, J.-C.; Shamir, R.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Standard versus Individualized Parenteral Nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef] [Green Version]
| Schofield Equation (kcal/Day) | |||
|---|---|---|---|
| males | <3 years | REE = 0.167 × W + 15.174 × H − 617.6 | 598.59 |
| 3–10 years | REE = 19.59 × W + 1.303 × H + 414.9 | 950.03 | |
| 10–18 years | REE = 16.25 × W + 1.372 × H + 705.8 | 1865.29 | |
| females | <3 years | REE = 16.252 × W + 10.232 × H − 413.5 | 567.58 |
| 3–10 years | REE = 16.969 × W + 1.618 × H + 371.2 | 888.56 | |
| 10–18 years | REE = 8.365 × W + 4.65 × H + 200 | 1467.31 | |
| Food Agriculture Organization (MJ/D) | |||
| males | <3 years | REE = 0.255 × W − 0.226 | 545.89 |
| 3–10 years | REE = 0.0949 × W + 2.07 | 948.37 | |
| 10–18 years | REE = 0.0732 × W + 2.72 | 1647.32 | |
| females | <3 years | REE = 0.255 × W − 0.214 | 558.32 |
| 3–10 years | REE = 0.0941 × W + 2.09 | 949.33 | |
| 10–18 years | REE = 0.051 × W + 3.12 | 1440.49 | |
| World Health Organization (WHO) | |||
| males | <3 years | 60.9 × W − 54 | 555 |
| 3–10 years | 22.7 × W + 495 | 949 | |
| 10–18 years | 17.5 × W + 651 | 1648.5 | |
| females | <3 years | 61 × W − 51 | 559 |
| 3–10 years | 22.5 × W + 499 | 949 | |
| 10–18 years | 22.2 × W + 746 | 2011.4 | |
| Essential | Semi-Essential (Conditionally Essential) | Nonessential |
|---|---|---|
| Arginine | Cysteine | Alanine |
| Histidine | Glutamine | Asparagine |
| Isoleucine | Hydroxyproline | Aspartate |
| Leucine | Proline | Glutamate |
| Lysine | Taurine | Glycine |
| Methionine | Serine | |
| Phenylalanine | Tyrosine | |
| Threonine | ||
| Tryptophan | ||
| Valine |
| Weight | mL/kg/d | mL/kg/h |
|---|---|---|
| A: the first 10 kg | 100 | 4 |
| B: between 10 and 20 kg | +50 mL/kg/d | +2 mL/kg/h |
| C: any kg above 20 kg | +25 mL/kg/d | +1 mL/kg/h |
| Daily calculation | A + B + C | A + B + C |
| Fat-Soluble Vitamins (Vitamin A, D, E, K) | |
|---|---|
| Vitamin A | 150–300 µg/kg/d |
| Vitamin D | 40–150 IU/kg/d up to 400–600 IU/d |
| Vitamin E | 2.8–3.5 mg/kg/d or 2.8–3.5 IU/kg/d 11 mg/d or 11 IU/d |
| Vitamin K | 10 µg/kg/d (or 200 µg/d |
| Water-soluble vitamins (Vitamin C, B vitamins) | |
| Vitamin C | 15–25 mg/kg/d up to 80 mg/d |
| Vitamin B1 (Thiamine) | 0.35–0.50 mg/kg/d up to 1.2 mg/d |
| Vitamin B2 (Riboflavin) | 0.15–0.2 mg/kg/d up to 1.4 mg/d |
| Vitamin B3 (Niacin) | 4–6.8 mg/kg/d up to 17 mg/d |
| Vitamin B5 (Pantothenic acid) | 2.5 mg/kg/d up to 5 mg/d |
| Vitamin B6 (Pyridoxine) | 0.15–0.2 mg/kg/d up to 1.0 mg/kg/d |
| Vitamin B7 (Biotin) | 5–8 µg/kg/d up to 20 µg/d |
| Vitamin B9 (Folic acid) | 56 mg/kg/d up to 140 mg/d |
| Vitamin B12 (Cyanocobalamin) | 0.3 µg/kg/d up to 1 µg/d |
| Trace minerals/elements | |
| Iron | 50–250 µg/kg/d up to 5 mg/d |
| Zinc | 50–500 µg/kg/d up to 5 mg/d |
| Copper | 20–40 µg/kg/d up to 0.5 mg/d |
| Iodine | 1–10 µg/kg/d |
| Selenium | 2–7 µg/kg/d up to 100 mg/d |
| Manganese | ≤1 µg/kg/d up to 50 mg/d |
| Molybdenum | 0.25–1 µg/kg/d up to 5 mg/d |
| Metoclopramide, Domperidone—agents for gastric motility improvement |
| Usually, 18–19 h of continuous administration via gastric tube with 5–6 h pause |
| Erythromycin—stimulate the bowel motility Consider subcutaneous naltrexone in patients on opioidsSuppository rectally applied |
| Oligomeric formula or peptide-based formula |
| Jejunal tube placement and continuous enteral feeding without the night pause |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratochvíl, M.; Klučka, J.; Klabusayová, E.; Musilová, T.; Vafek, V.; Skříšovská, T.; Djakow, J.; Havránková, P.; Osinová, D.; Štourač, P. Nutrition in Pediatric Intensive Care: A Narrative Review. Children 2022, 9, 1031. https://doi.org/10.3390/children9071031
Kratochvíl M, Klučka J, Klabusayová E, Musilová T, Vafek V, Skříšovská T, Djakow J, Havránková P, Osinová D, Štourač P. Nutrition in Pediatric Intensive Care: A Narrative Review. Children. 2022; 9(7):1031. https://doi.org/10.3390/children9071031
Chicago/Turabian StyleKratochvíl, Milan, Jozef Klučka, Eva Klabusayová, Tereza Musilová, Václav Vafek, Tamara Skříšovská, Jana Djakow, Pavla Havránková, Denisa Osinová, and Petr Štourač. 2022. "Nutrition in Pediatric Intensive Care: A Narrative Review" Children 9, no. 7: 1031. https://doi.org/10.3390/children9071031
APA StyleKratochvíl, M., Klučka, J., Klabusayová, E., Musilová, T., Vafek, V., Skříšovská, T., Djakow, J., Havránková, P., Osinová, D., & Štourač, P. (2022). Nutrition in Pediatric Intensive Care: A Narrative Review. Children, 9(7), 1031. https://doi.org/10.3390/children9071031