Predictors of Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: A Retrospective Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Study Design and Setting
2.3. Patients’ Recruitment
2.4. Study Definitions
2.5. Institutional Guidelines and Protocols
2.6. Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 68, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Jastaniah, W. Epidemiology of sickle cell disease in Saudi Arabia. Ann. Saudi Med. 2011, 31, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality In Sickle Cell Disease—Life Expectancy and Risk Factors for Early Death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Powars, D.; Weidman, J.A.; Odom-Maryon, T.; Niland, J.C.; Johnson, C. Sickle cell chronic lung disease: Prior morbidity and the risk of pulmonary failure. Medicine 1988, 67, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and Outcomes of the Acute Chest Syndrome in Sickle Cell Disease. N. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef] [Green Version]
- Ballas, S.K.; Lieff, S.; Benjamin, L.J.; Dampier, C.D.; Heeney, M.M.; Hoppe, C.; Johnson, C.S.; Rogers, Z.R.; Smith-Whitley, K.; Wang, W.C.; et al. Definitions of the phenotypic manifestations of sickle cell disease. Am. J. Hematol. 2009, 85, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Okubo, Y.; Handa, A. Acute chest syndrome among children hospitalized with vaso-occlusive crisis: A nationwide study in the United States. Pediatr. Blood Cancer 2018, 65, 1–7. [Google Scholar] [CrossRef]
- Patterson, G.D.; Mashegu, H.; Rutherford, J.; Seals, S.; Josey, D.; Karlson, C.; McNaull, M.; May, W.; Carroll, C.; Barr, F.E.; et al. Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: Clinical Features and Risk Factors. J. Pediatr. Hematol. 2018, 40, 51–55. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Rodeghier, M.; Cohen, R.; Kirkham, F.; Rosen, C.L.; Roberts, I.; Cooper, B.; Stocks, J.; Wilkey, O.; Inusa, B.; et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am. J. Hematol. 2014, 89, E212–E217. [Google Scholar] [CrossRef] [Green Version]
- Wales, P.; Carver, E.; Crawford, M.; Kim, P. Acute chest syndrome after abdominal surgery in children with sickle cell disease: Is a laparoscopic approach better? J. Pediatr. Surg. 2001, 36, 718–721. [Google Scholar] [CrossRef]
- Buchanan, I.D.; Woodward, M.; Reed, G.W. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatr. Blood Cancer 2005, 45, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, E.; Jacobson, S.; Joshi, P.; Koren, G. Systemic exposure to morphine and the risk of acute chest syndrome in sickle cell disease. Clin. Pharmacol. Ther. 2004, 75, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Hankins, J.; Jeng, M.; Harris, S.; Li, C.-S.; Liu, T.; Wang, W. Chronic Transfusion Therapy for Children With Sickle Cell Disease and Recurrent Acute Chest Syndrome. J. Pediatr. Hematol. 2005, 27, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.T.; Wright, E.; Abboud, M.; Berman, B.; Files, B.; Scher, C.D.; Styles, L.; Adams, R.J. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J. Pediatr. 2001, 139, 785–789. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Hydroxyurea for Treatment of Pediatric Patients with Sickle Cell Anemia. U.S. Food and Drug Administration. 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-hydroxyurea-treatment-pediatric-patients-sickle-cell-anemia (accessed on 16 May 2022).
- Knight-Madden, J.M.; Forrester, T.S.; Lewis, N.A.; Greenough, A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax 2005, 60, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.H.; Macklin, E.; Strunk, R.C.; DeBaun, M.R. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood 2006, 108, 2923–2927. [Google Scholar] [CrossRef] [Green Version]
- Thornburg, C.D.; Files, B.A.; Luo, Z.; Miller, S.T.; Kalpatthi, R.; Iyer, R.; Seaman, P.; Lebensburger, J.; Alvarez, O.; Thompson, B.; et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood 2012, 120, 4304–4310. [Google Scholar] [CrossRef] [Green Version]
- Yawn, B.P.; Buchanan, G.R.; Afenyi-Annan, A.N.; Ballas, S.K.; Hassell, K.L.; James, A.H.; Jordan, L.; Lanzkron, S.; Lottenberg, R.; Savage, W.J.; et al. Management of Sickle Cell Disease: Summary of the 2014 Evidence-Based Report by Expert Panel Members. JAMA 2014, 312, 1033–1048. [Google Scholar] [CrossRef]
- Styles, L.A.; Vichinsky, E. Effects of a long-term transfusion regimen on sickle cell-related illnesses. J. Pediatr. 1994, 125 Pt 1, 909–911. [Google Scholar] [CrossRef]
- Dolatkhah, R.; Dastgiri, S. Blood transfusions for treating acute chest syndrome in people with sickle cell disease. Cochrane Database Syst. Rev. 2020, 1, CD007843. [Google Scholar] [CrossRef]
- Leibovitch, J.N.; Tambe, A.V.; Cimpeanu, E.; Poplawska, M.; Jafri, F.; Dutta, D.; Lim, S.H. L-glutamine, crizanlizumab, voxelotor, and cell-based therapy for adult sickle cell disease: Hype or hope? Blood Rev. 2022, 53, 100925. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.M.; Fitzhugh, C.D.; Tisdale, J.F. Allogeneic hematopoietic stem cell transplantation for sickle cell disease: The time is now. Blood 2011, 118, 1197–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, A.A.; Shash, H.A.; Almajid, A.N.; Binammar, A.A.; Almusabeh, H.A.; Alshaqaq, H.M.; Al-Qahtani, M.H.; Albuali, W.H. Acute chest syndrome in pediatric sickle cell disease: A 19-year tertiary-center experience. Ann. Thorac. Med. 2022, in press. [Google Scholar]

| Study Variables | Total Population (n = 42) | Patients with Single ACS Episode (n = 17) | Patients with Recurrent ACS Episodes (n = 25) | p-Value |
|---|---|---|---|---|
| Age at the time of SCD Diagnosis, median (IQR) *, months | 8 (6–24) | 8.5 (5.25–36) | 8 (6–24) | 0.911 a |
| Male:Female ratio, n (%) | 22:20 (52.4%:47.6%) | 10:7 (58.8%:41.2%) | 12:13 (48%:52%) | 0.491 b |
| Hgb SS, n (%) | 29 (69%) | 12 (70.6%) | 17 (68%) | 0.859 b |
| Hgb Sβ0 thalassemia, n (%) | 9 (21.4%) | 3 (17.6%) | 6 (24%) | 0.490 b |
| Hgb Sα thalassemia, n (%) | 4 (9.5%) | 2 (11.8%) | 2 (8%) | 1 b |
| Age at the time of first ACS diagnosis, mean ± SD | 6.62 ± 3.38 | 8.67 ± 2.54 | 5.16 ± 3.13 | <0.001 **c |
| Number of acute anemia/year, mean ± SD | 3.64 ± 1.74 | 2.88 ± 1.46 | 4.67 ± 1.63 | 0.051 c |
| Number of VOC/year, median (IQR) | 2 (1–3) | 2 (1.25–3) | 2 (1–3) | 0.899 a |
| SCD-related hospitalizations/year, median (IQR) | 3 (2–4) | 2 (1–4) | 4 (2–7) | 0.026 **a |
| Comorbidities: | ||||
| G6PD deficiency, n (%) | 9 (21.4%) | 4 (23.5%) | 5 (20%) | 1 b |
| Asthma, n (%) | 11 (26.2%) | 2 (11.8%) | 9 (36%) | 0.151 b |
| CVS diseases, n (%) d | 2 (4.8%) | 0 | 2 (8%) | 0.506 b |
| Endocrine diseases, n (%) e | 3 (7.1%) | 1 (5.9%) | 2 (8%) | 1 b |
| Developmental delay, n (%) | 2 (4.8%) | 0 | 2 (8%) | 0.506 b |
| OSA, n (%) | 1 (2.4%) | 0 | 1 (4%) | 1 b |
| Miscellaneous, n (%) f | 4 (9.52%) | 0 | 4 (9.52%) | 0.134 b |
| ≥ 1 comorbidities, n (%) | 22 (52.4%) | 7 (41.2%) | 15 (60%) | 0.231 b |
| Medications before ACS episodes: | ||||
| Hydroxyurea therapy at baseline, n (%) | 1 (2.4%) | 0 | 1 (4%) | 1 b |
| Folic acid, n (%) | 39 (92.9%) | 15 (88.2%) | 24 (96%) | 0.556 b |
| Penicillin V, n (%) * | 28 (66.7%) | 14 (82.4%) | 14 (56%) | 0.075 b |
| Regular transfusion, n (%) * | 2 (4.8%) | 1 (5.9%) | 1 (4%) | 1 b |
| Ventolin, n (%) * | 5 (11.9%) | 2 (11.8%) | 3 (12%) | 1 b |
| Aspirin, n (%) * | 1 (2.4%) | 0 | 1 (4%) | 1 b |
| Amlodipine, n (%) * | 1 (2.4%) | 0 | 1 (4%) | 1 b |
| Study Variables | Total Population (n = 91) | Non-Recurrent ACS (n = 17) | Recurrent ACS (n = 74) | p-Value |
|---|---|---|---|---|
| Age at time of all ACS episodes’ diagnoses, mean ± SD | 7.18 ± 3.38 | 9 ± 2.76 | 6.76 ± 3.39 | 0.013 **a |
| ACS episodes required PICU admission, n (%) | 9 (9.9%) | 2 (11.8%) | 7 (9.5%) | 0.673 c |
| Hydroxyurea before each ACS episode, n (%) | 10 (11%) | 0 | 10 (13.5%) | 0.2 c |
| Clinical features at hospital presentation: | ||||
| SOB, n (%) | 47 (51.6%) | 12 (70.6%) | 35 (47.3%) | 0.083 c |
| Fever, n (%) | 64 (70.3%) | 11 (64.7%) | 53 (71.6%) | 0.574 c |
| Cough, n (%) | 64 (70.3%) | 10 (58.8%) | 54 (73%) | 0.249 c |
| Chest pain, n (%) | 30 (33%) | 7 (41.2%) | 23 (31.1%) | 0.425 c |
| Extremity pain, n (%) | 18 (19.8%) | 5 (29.4%) | 13 (17.6%) | 0.314 c |
| Back pain, n (%) | 15 (16.5%) | 6 (35.3%) | 9 (12.2%) | 0.031 **c |
| URTI symptoms, n (%) | 20 (22%) | 3 (17.6%) | 17 (23%) | 0.755 c |
| GI symptoms, n (%) | 15 (16.5%) | 4 (23.5%) | 11 (14.9%) | 0.468 c |
| Others, n (%) d | 6 (6.6%) | 1 (5.9%) | 5 (6.8%) | 1 c |
| Vital signs: | ||||
| Heart rate, mean ± SD | 118.78 ± 22.09 bpm | 117.81 ± 25.74 bpm | 119.02 ± 21.31 bpm | 0.847 a |
| Respiratory rate, median (IQR) | 32 (24–42) bpm | 33 (25–42.5) bpm | 32 (22–41) bpm | 0.679 b |
| Temperature, mean ± SD | 37.91 ± 0.97 °C | 37.54 ± 0.94 °C | 37.99 ± 0.96 °C | 0.105 a |
| SBP, mean ± SD | 104.62 ± 11.42 mmHg | 102 ± 7.36 mmHg | 105.29 ± 12.21 mmHg | 0.323 a |
| DBP, median (IQR) | 59 (55–62) mmHg | 61 (58.5–66) mmHg | 59 (55–60) mmHg | 0.507 b |
| MAP, mean ± SD | 77.08 ± 8.8 mmHg | 76 ± 6.44 mmHg | 77.36 ± 9.34 mmHg | 0.597 a |
| SpO2, median (IQR) | 96 (89–99)% | 99 (88–99)% | 95.5 (89.75–97.25)% | 0.471 b |
| Chest examination: | ||||
| Wheezing, n (%) | 10 (11%) | 1 (5.9%) | 9 (12.2%) | 0.681 c |
| Crackles, n (%) | 44 (48.4%) | 5 (29.4%) | 39 (52.7%) | 0.083 c |
| Reduced breath sound, n (%) | 33 (36.3%) | 8 (47.1%) | 25 (33.8%) | 0.305 c |
| Respiratory distress, n (%) e | 17 (18.7%) | 1 (5.9%) | 16 (21.6%) | 0.179 c |
| Study Variables | Total Population (n = 42) | Patients with Single ACS Episode (n = 17) | Patients with Recurrent ACS Episodes (n = 25) | p-Value |
|---|---|---|---|---|
| WBC count, mean ± SD, (n = 29) | 14.1 ± 6.42 k/uL | 11.13 ± 3.73 k/uL | 16.87 ± 7.24 k/uL | 0.013 **a |
| Neutrophils, mean ± SD, (n = 23) | 40.59 ± 14.56 % | 44.11 ± 13.43% | 35.11 ± 15.54% | 0.155 a |
| Eosinophils, median (IQR), (n = 27) | 3 (1.9–4%) | 2 (1.1–4.1)% | 3 (2.08–4)% | 0.581 b |
| Lymphocytes, mean ± SD, (n = 27) | 43.69 ± 16.24% | 40.98 ± 16% | 47.1 ± 16.58% | 0.342 a |
| Hemoglobin, mean ± SD, (n = 31) | 8.28 ± 0.88 g/dL | 8.57 ± 0.9 g/dL | 8 ± 0.80 g/dL | 0.07 a |
| MCV, mean ± SD, (n = 31) | 76.58 ± 13.11 fL | 70.91 ± 15.47 fL | 81.89 ± 7.64 fL | 0.017 **a |
| MCH, mean ± SD, (n = 31) | 30.13 ± 13.51 pg | 29.32 ± 15.32 pg | 30.89 ± 12.04 pg | 0.751 a |
| RBC count, mean ± SD, (n = 31) | 3.2 ± 0.732 Mil/uL | 3.52 ± 0.89 Mil/uL | 2.91 ± 0.39 Mil/uL | 0.024 **a |
| Hematocrit, mean ± SD, (n = 31) | 24.58 ± 2.95% | 25.78 ± 3.48 % | 23.45 ± 1.83% | 0.026 **a |
| Reticulocyte count, mean ± SD, (n = 31) | 9.57 ± 4.46% | 7.81 ± 3.95 % | 11.21 ± 4.66% | 0.031 **a |
| Platelets, mean ± SD, (n = 30) | 410.9 ± 184.15 k/uL | 418.4 ± 220.24 k/uL | 403.4 ± 147.03 k/uL | 0.828 a |
| BUN, mean ± SD, (n = 16) | 7.47 ± 2.69 mg/dL | 8.38 ± 3.06 mg/dL | 6.56 ± 2.06 mg/dL | 0.186 a |
| Creatinine, median (IQR), (n = 15) | 0.3 (0.3–4) mg/dL | 0.4 (0.28–0.4) mg/dL | 0.3 (0.3–0.4) mg/dL | 0.536 b |
| Total bilirubin, mean ± SD, (n = 12) | 2.54 ± 1.61 mg/dL | 1.25 ± 0.48 mg/dL | 3.19 ± 1.59 mg/dL | 0.011 **a |
| Direct bilirubin, median (IQR), (n = 12) | 0.3 (0.293–0.475) mg/dL | 0.25 (0.2–0.3) mg/dL | 0.4 (0.3–0.5) mg/dL | 0.008 **b |
| AST, median (IQR), (n = 12) | 48 (41.5–76.75) U/L | 66 (29.75–149.5) U/L | 45.5 (41.5–58.5) U/L | 0.570 b |
| ALT, median (IQR), (n = 12) | 29.5 (24.75–44.375) U/L | 40 (27.75–96.5) U/L | 28.5 (21–32.63) U/L | 0.154 b |
| Alkaline phosphates, mean ± SD, (n = 12) | 175.5 ± 40.42 U/L | 171.5 ± 31.93 U/L | 177.5 ± 46 U/L | 0.821 a |
| LDH, mean ± SD, (n = 12) | 519.5 ± 184.55 U/L | 434.5 ± 202.69 U/L | 562 ± 172.39 U/L | 0.279 a |
| Study Variables | Total Population (n = 91) | Single ACS Episode (n = 17) | Recurrent ACS Episodes (n = 74) | p-Value |
|---|---|---|---|---|
| Hospital LOS, median (IQR) * | 8 (5–10.25) (n = 86) | 9 (6–14) (n = 15) | 7 (5–10) (n = 71) | 0.108 a |
| PICU LOS, median (IQR) | 4 (3–5.5) (n = 9) | 21.5 (12.25–30.75) (n = 2) | 4 (3–5) (n = 7) | 0.667 a |
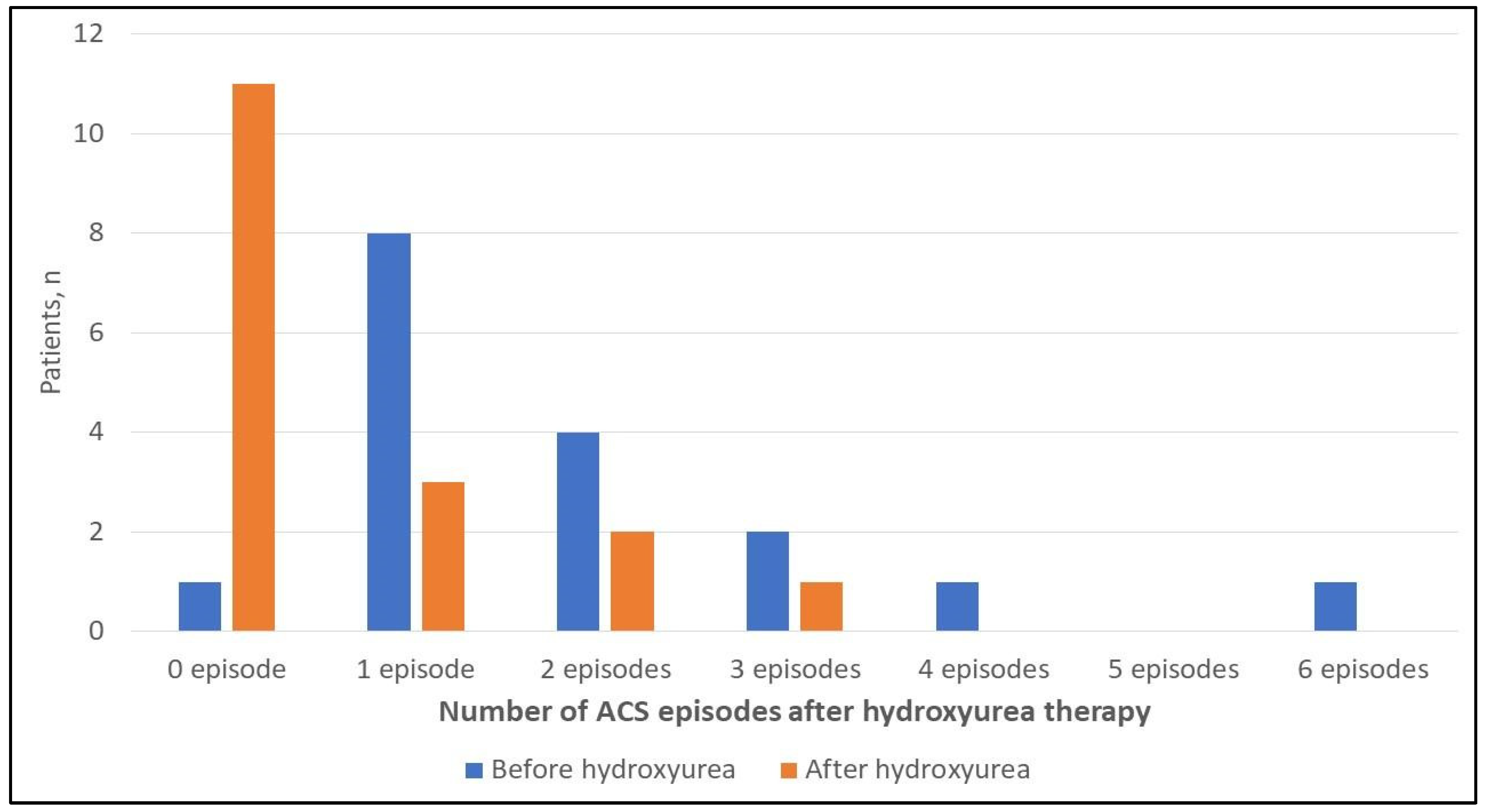
| Number of ACS episodes before initiating hydroxyurea, median (IQR) | 1 (1–2.5) (n = 17) | 1 (1–1) (n = 5) | 2 (1–3) (n = 12) | 0.021 **b |
| Number of ACS episodes after initiating hydroxyurea, median (IQR) | 0 (0–1) (n = 17) | 0 (0–0) (n = 5) | 0.5 (0–1.75) (n = 12) |
| Study Variables | UOR (95% CI) | p-Value |
|---|---|---|
| Age at time of first ACS diagnosis (per 1-year increase), (n = 42/42) | 0.672 (0.515–0.876) | 0.003 ** |
| Age at time of all ACS episodes’ diagnoses (per 1-year increase), (n = 91/91) | 0.805 (0.674–0.963) | 0.017 ** |
| SCD-related hospitalizations/year (per 1 hospitalization increase), (n = 37/42) | 1.639 (1.027–2.616) | 0.038 ** |
| Baseline WBC count (per 1-unit increase), (n = 29/42) | 1.267 (1.027–1.564) | 0.028 ** |
| Baseline MCV (per 1-unit increase), (n = 31/42) | 1.108 (1.010–1.215) | 0.031 ** |
| Baseline RBC count (per 1-unit increase), (n = 31/42) | 0.211(0.049–0.913) | 0.037 ** |
| Baseline hematocrit (per 1-unit increase), (n = 31/42) | 0.714 (0.519–0.983) | 0.039 ** |
| Baseline reticulocyte count (per 1-unit increase), (n = 31/42) | 1.253 (1.005–1.562) | 0.045 ** |
| Back pain, (n = 91/91) | ||
| Reference | - |
| 0.254 (0.075–0.855) | 0.027 ** |
| Neutrophil at time of ACS diagnosis (per 1-unit increase), (n = 64/91) | 0.957 (0.917–0.998) | 0.041 ** |
| RBC count 24 h before discharge (per 1-unit increase), (n = 32/91) | 0.029 (0.002–0.514) | 0.016 ** |
| MCV at time of admission (per 1-unit increase), (n = 89/91) | 1.084 (1.021–1.150) | 0.008 ** |
| MCV at time of diagnosis (per 1-unit increase), (n = 86/91) | 1.087 (1.020–1.157) | 0.010 ** |
| MCV 24 h before discharge (per 1-unit increase),(n = 32/91) | 1.137 (1.005–1.287) | 0.042 ** |
| Qualitative CRP at time of admission, (n = 74/91): | ||
| Reference | - |
| 9.333 (1.919–45.386) | 0.006 ** |
| Use of NSAIDs, (n = 91/91): | ||
| Reference | - |
| 0.278 (0.092–0.840) | 0.023 ** |
| Use of clarithromycin, (n = 91/91): | ||
| Reference | - |
| 0.064 (0.006–0.660) | 0.021 ** |
| Use of hydroxyurea therapy, (n = 91): | ||
| Reference | - |
| 0.465 (0.138–1.562) | 0.215 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, A.A.; Shash, H.A.; Almajid, A.N.; Binammar, A.A.; Almusabeh, H.A.; Alshaqaq, H.M.; Al-Qahtani, M.H.; Albuali, W.H. Predictors of Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: A Retrospective Case-Control Study. Children 2022, 9, 894. https://doi.org/10.3390/children9060894
Yousef AA, Shash HA, Almajid AN, Binammar AA, Almusabeh HA, Alshaqaq HM, Al-Qahtani MH, Albuali WH. Predictors of Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: A Retrospective Case-Control Study. Children. 2022; 9(6):894. https://doi.org/10.3390/children9060894
Chicago/Turabian StyleYousef, Abdullah A., Hwazen A. Shash, Ali N. Almajid, Ammar A. Binammar, Hamza Ali Almusabeh, Hassan M. Alshaqaq, Mohammad H. Al-Qahtani, and Waleed H. Albuali. 2022. "Predictors of Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: A Retrospective Case-Control Study" Children 9, no. 6: 894. https://doi.org/10.3390/children9060894
APA StyleYousef, A. A., Shash, H. A., Almajid, A. N., Binammar, A. A., Almusabeh, H. A., Alshaqaq, H. M., Al-Qahtani, M. H., & Albuali, W. H. (2022). Predictors of Recurrent Acute Chest Syndrome in Pediatric Sickle Cell Disease: A Retrospective Case-Control Study. Children, 9(6), 894. https://doi.org/10.3390/children9060894









