Does Focal Osteolysis in a PRECICE Stryde Intramedullary Lengthening Nail Resolve after Explantation?
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Stainless-Steel Intramedullary Lengthening Nail
2.3. Patient Selection
2.4. Intramedullary Nail Implantation and Explantation Technique
2.5. Outcomes of Interest
2.6. Statistical Analyses
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Adverse Tissue Reactions Prior to Explantation
3.3. Adverse Tissue Reactions Post-Explantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, J.E.; Davis, L.E. Leg Length Discrepancy: The Natural History (And What Do We Really Know). J. Pediatr. Orthop. 2019, 39, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, L.; Specht, S.C.; Standard, S.C.; Herzenberg, J.E. Internal Lengthening Device for Congenital Femoral Deficiency and Fibular Hemimelia. Clin. Orthop. Relat. Res. 2014, 472, 3860–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ramirez, A.; Thacker, M.M.; Becerra, L.C.; Riddle, E.C.; MacKenzie, W.G. Limb length discrepancy and congenital limb anomalies in fibular hemimelia. J. Pediatr. Orthop. Part B 2010, 19, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues. Part, I. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. 1989, 238, 249–281. [Google Scholar] [CrossRef]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. 1989, 239, 263–285. [Google Scholar] [CrossRef]
- Rozbruch, S.R.; Kleinman, D.; Fragomen, A.T.; Ilizarov, S. Limb lengthening and then insertion of an intramedullary nail: A case-matched comparison. Clin. Orthop. Relat. Res. 2008, 466, 2923–2932. [Google Scholar] [CrossRef] [Green Version]
- Paley, D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin. Orthop. Relat. Res. 1990, 250, 81–104. [Google Scholar] [CrossRef]
- Baumgart, R.; Betz, A.; Schweiberer, L. A fully implantable motorized intramedullary nail for limb lengthening and bone transport. Clin. Orthop. Relat. Res. 1997, 343, 135–143. [Google Scholar] [CrossRef]
- Baumgart, R.; Zeiler, C.; Kettler, M.; Weiss, S.; Schweiberer, L. Fully implantable intramedullary distraction nail in shortening deformity and bone defects. Spectrum of indications. Orthopade 1999, 28, 1058–1065. [Google Scholar]
- Morrison, T.A.; Sontich, J.K. Premature consolidation with resultant implant failure using PRECICE femoral nail lengthening. JBJS Case Connect. 2016, 6, e2. [Google Scholar] [CrossRef]
- URGENT RECALL NOTIFICATION PRECICE STRYDE, PRECICE PLATE AND PRECICE BONE TRANSPORT. 2021. Available online: https://www.nuvasive.com/wp-content/uploads/2021/02/NSO-Precice-FSN-United-States-Biodur.pdf (accessed on 25 January 2022).
- Gittings, D.J.; Dattilo, J.R.; Hardaker, W.; Sheth, N.P. Evaluation and treatment of femoral osteolysis following total hip arthroplasty. JBJS Rev. 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.Y.; Yoon, T.R. Recent updates for biomaterials used in total hip arthroplasty. Biomater. Res. 2018, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Sheth, N.P.; Rozell, J.C.; Paprosky, W.G. Evaluation and Treatment of Patients with Acetabular Osteolysis after Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2019, 27, 258–267. [Google Scholar] [CrossRef]
- Doorn, P.F.; Campbell, P.A.; Amstutz, H.C. Metal versus polyethylene wear particles in total hip replacements: A review. Clin. Orthop. Relat. Res. 1996, 29, S206–S216. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, Y.; Revell, P.A.; Kobayashi, A.; Al-Saffar, N.; Scott, G.; Freeman, M.A.R. Wear particulate species and bone loss in failed total joint arthroplasties. Clin. Orthop. Relat. Res. 1997, 40, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Howie, D.W. Tissue response in relation to type of wear particles around failed hip arthroplasties. J. Arthroplast. 1990, 5, 337–348. [Google Scholar] [CrossRef]
- Jones, D.M.; Marsh, J.L.; Nepola, J.V.; Jacobs, J.J.; Skipor, A.K.; Urban, R.M.; Gilbert, J.L.; Buckwalter, J.A. Focal osteolysis at the junctions of a modular stainless-steel femoral intramedullary nail. JBJS 2001, 83, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Sax, O.C.; Molavi, D.W.; Herzenberg, J.E.; Standard, S.C.; McClure, P.K. Biopsy Proven Focal Osteolysis in a Stainless-Steel Limb-Lengthening Device: A Report of Three Cases. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21. [Google Scholar] [CrossRef]
- Frommer, A.; Roedl, R.; Gosheger, G.; Hasselmann, J.; Fuest, C.; Toporowski, G.; Laufer, A.; Tretow, H.; Schulze, M.; Vogt, B. Focal osteolysis and corrosion at the junction of precice stryde intramedullary lengthening device preliminary clinical, radiological, and metallurgic analysis of 57 lengthened segments. Bone Jt. Res. 2021, 10, 425–436. [Google Scholar] [CrossRef]
- Iliadis, A.D.; Wright, J.; Stoddart, M.T.; Goodier, W.D.; Calder, P. Early results from a single centre’s experience with the STRYDE nail: A cause for concern? Bone Jt. J. 2021, 103, 1168–1172. [Google Scholar] [CrossRef]
- Jellesen, M.S.; Lomholt, T.N.; Hansen, R.Q.; Mathiesen, T.; Gundlach, C.; Kold, S.; Nygaard, T.; Mikuzis, M.; Olesen, U.K.; Rölfing, J.D. The STRYDE limb lengthening nail is susceptible to mechanically assisted crevice corrosion: An analysis of 23 retrieved implants. Acta Orthop. 2021, 92, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Rölfing, J.D.; Kold, S.; Nygaard, T.; Mikuzis, M.; Brix, M.; Faergemann, C.; Gottliebsen, M.; Davidsen, M.; Petruskevicius, J.; Olesen, U.K. Pain, osteolysis, and periosteal reaction are associated with the STRYDE limb lengthening nail: A nationwide cross-sectional study. Acta Orthop. 2021, 92, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Sax, O.C.; Conway, J.D.; Assayag, M.; Standard, S.C.; Herzenberg, J.E.; McClure, P.K. Risk factors for focal osteolysis in a stainless-steel limb-lengthening device. J. Limb. Lengthening Reconstr. 2021, 7, 19–25. [Google Scholar] [CrossRef]
- Burghardt, R.D.; Manzotti, A.; Bhave, A.; Paley, D.; Herzenberg, J.E. Tibial lengthening over intramedullary nails: A matched case comparison with Ilizarov tibial lengthening. Bone Jt. Res. 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, R.D.; Herzenberg, J.E.; Specht, S.C.; Paley, D. Mechanical failure of the intramedullary skeletal kinetic distractor in limb lengthening. JBJS 2011, 93, 639–643. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Jasty, M.; Harris, W.H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. Bone Jt. Surg 1992, 74, 849–863. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Roebuck, K.A.; Archibeck, M.; Hallab, N.J.; Glant, T.T. Osteolysis: Basic science. Clin. Orthop. Relat. Res. 2001, 393, 71–77. [Google Scholar] [CrossRef]
- Iobst, C.A.; Frost, M.W.; Rölfing, J.D.; Rahbek, O.; Bafor, A.; Duncan, M.; Kold, S.; Helenius, I.J.; Krieg, A.H.; van der Heijden, L.; et al. Radiographs of 366 removed limb-lengthening nails reveal differences in bone abnormalities between different nail types. Bone Jt. J. 2021, 103, 1731–1735. [Google Scholar] [CrossRef]
- Hothi, H.; Bergiers, S.; Henckel, J.; Iliadis, A.D.; Goodier, W.D.; Wright, J.; Skinner, J.; Calder, P.; Hart, A.J. Analysis of retrieved STRYDE nails. Bone Jt. Open 2021, 2, 599–610. [Google Scholar] [CrossRef]
| Mean Age | 15.6 |
|---|---|
| Gender (M, F) | 43 M, 14 F |
| Laterality (L, R) | 37 R, 20 L |
| Bone (Femur, Tibia) | 38 Femurs, 19 Tibias |
| Average Clinical Follow-up Time (Months) | 5.6 |
| Etiology | 48 Congenital, 5 Idiopathic, 4 Traumatic |
| Average Weight (kg) | 63 |
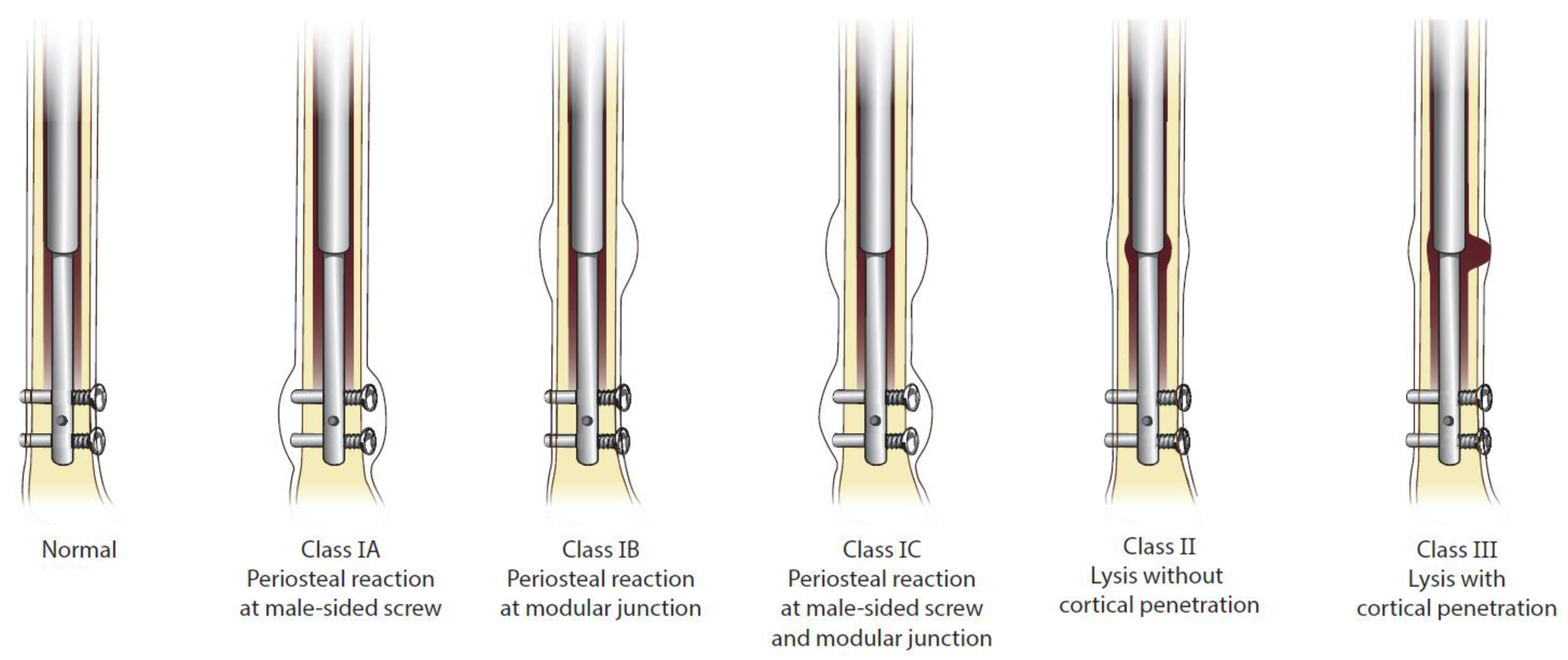
| Patient * | Osteolysis Onset (mo) | Time to Explantation (mo) | Periosteal Reaction Class Prior to Explanation (1A/1B/1C) | Osteolysis Class Prior to Explanation (II/III) |
|---|---|---|---|---|
| 1 | 13 | 18 | 1A | II |
| 2 | 10 | 14 | 1C | II |
| 3 | 20 | 22 | 1C | II |
| 4 | 9 | 33 | 1C | II |
| 5 | 6 | Not explanted/lost to f/u | 1A | II |
| 6 | 12 | 20 | 1A | II |
| 7 | 9 | 11 | 1A | II |
| 8 | 2 | 5 | 1C | III |
| 9 | 14 | 19 | 1C | II |
| 10 | 8 | 16 | 1C | II |
| 11 | 10 | 23 | 1A | II |
| 12 | 23 | 24 | 1B | II |
| 13 | 5 | 11 | 1C | II |
| 14 | 6 | 7 | 1B | II |
| 15 | 6 | 7 | 1B | II |
| Patient * | Last Clinical f/u (mo) | Last Clinical f/u Osteolysis Classification (II/III) | Osteolysis Resolution |
|---|---|---|---|
| 1 | 9 | II | Complete |
| 2 | 9 | II | Complete |
| 3 | 6 | II | Partial |
| 4 | 13 | II | Complete |
| 5 | No f/u | II | n/a |
| 6 | 14 | II | Complete |
| 7 | not explanted | ||
| 8 | 7 | III | Partial |
| 9 | No f/u | II | n/a |
| 10 | No f/u | II | n/a |
| 11 | No f/u | II | n/a |
| 12 | No f/u | II | n/a |
| 13 | No f/u | II | n/a |
| 14 | 10 | II | Complete |
| 15 | 12 | II | Complete |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sax, O.C.; Hlukha, L.P.; Kowalewski, K.A.; Herzenberg, J.E.; McClure, P.K. Does Focal Osteolysis in a PRECICE Stryde Intramedullary Lengthening Nail Resolve after Explantation? Children 2022, 9, 860. https://doi.org/10.3390/children9060860
Sax OC, Hlukha LP, Kowalewski KA, Herzenberg JE, McClure PK. Does Focal Osteolysis in a PRECICE Stryde Intramedullary Lengthening Nail Resolve after Explantation? Children. 2022; 9(6):860. https://doi.org/10.3390/children9060860
Chicago/Turabian StyleSax, Oliver C., Larysa P. Hlukha, Kyle A. Kowalewski, John E. Herzenberg, and Philip K. McClure. 2022. "Does Focal Osteolysis in a PRECICE Stryde Intramedullary Lengthening Nail Resolve after Explantation?" Children 9, no. 6: 860. https://doi.org/10.3390/children9060860
APA StyleSax, O. C., Hlukha, L. P., Kowalewski, K. A., Herzenberg, J. E., & McClure, P. K. (2022). Does Focal Osteolysis in a PRECICE Stryde Intramedullary Lengthening Nail Resolve after Explantation? Children, 9(6), 860. https://doi.org/10.3390/children9060860





