Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
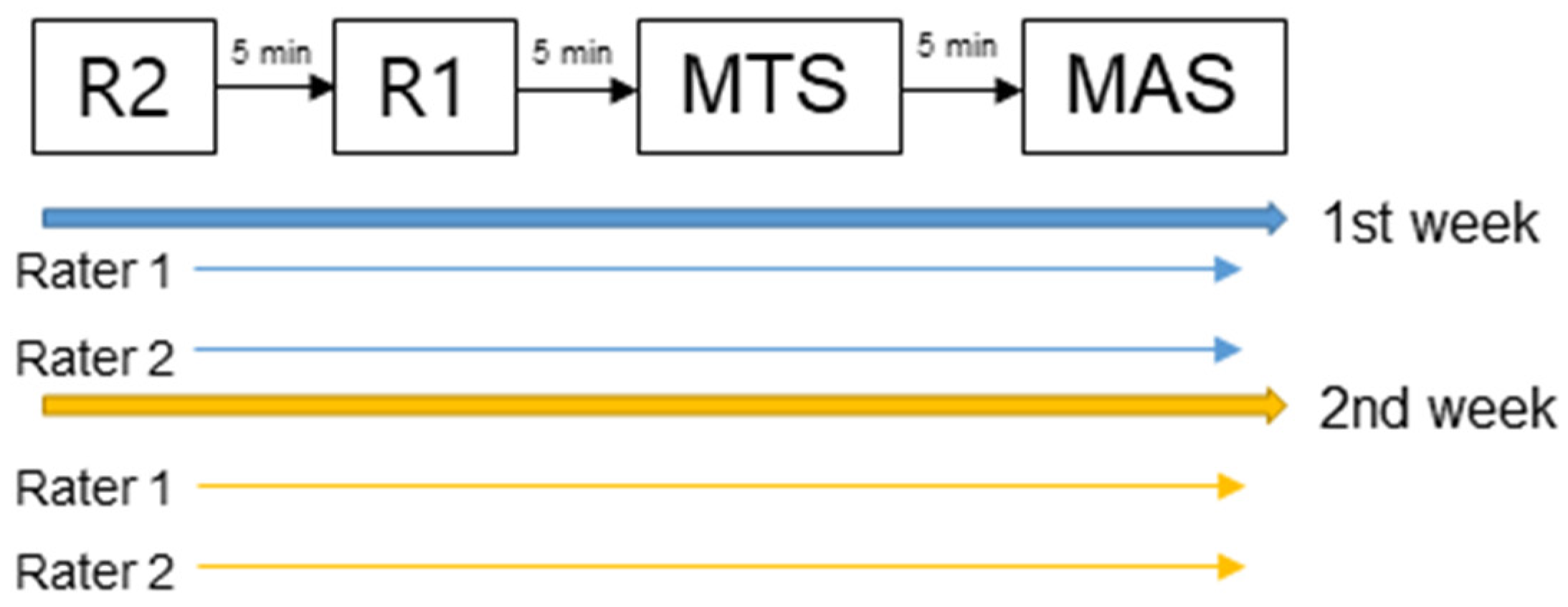
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, J.; Soo, B.; Graham, H.K.; Boyd, R.N.; Reid, S.; Lanigan, A.; Wolfe, R.; Reddihough, D.S. Cerebral palsy in victoria: Motor types, topography and gross motor function. J. Paediatr. Child Health 2005, 41, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, S.K.; Park, E.S. The effect of botulinum toxin injections on gross motor function for lower limb spasticity in children with cerebral palsy. Toxins 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Franzén, M.; Hägglund, G.; Alriksson-Schmidt, A. Treatment with botulinum toxin a in a total population of children with cerebral palsy-a retrospective cohort registry study. BMC Musculoskelet. Disord. 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.A.; Dean, C.M.; Crosbie, J.; Shepherd, R.B.; Lewis, J. Evaluation of spasticity in children with cerebral palsy using ashworth and tardieu scales compared with laboratory measures. J. Child Neurol. 2010, 25, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Alibiglou, L.; Rymer, W.Z.; Harvey, R.L.; Mirbagheri, M.M. The relation between ashworth scores and neuromechanical measurements of spasticity following stroke. J. Neuroeng. Rehabil. 2008, 5, 1–14. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Price, C.I.; Barnes, M.P.; Johnson, G.R. A biomechanical investigation into the validity of the modified ashworth scale as a measure of elbow spasticity. Clin. Rehabil. 2003, 17, 290–294. [Google Scholar] [CrossRef]
- Fosang, A.L.; Galea, M.P.; McCoy, A.T.; Reddihough, D.S.; Story, I. Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 664–670. [Google Scholar] [CrossRef]
- Clopton, N.; Dutton, J.; Featherston, T.; Grigsby, A.; Mobley, J.; Melvin, J. Interrater and intrarater reliability of the modified ashworth scale in children with hypertonia. Pediatric Phys. Ther. 2005, 17, 268–274. [Google Scholar] [CrossRef]
- Yam, W.K.L.; Leung, M.S.M. Interrater reliability of modified ashworth scale and modified tardieu scale in children with spastic cerebral palsy. J. Child Neurol. 2006, 21, 1031–1035. [Google Scholar] [CrossRef]
- Numanog, L.; Günel, M. Intraobserver reliability of modified ashworth scale and modified tardieu scale in the assessment of spasticity in children with cerebral palsy. Acta Orthop. Traumatol. Turc. 2011, 46, 196–200. [Google Scholar] [CrossRef]
- Mutlu, A.; Livanelioglu, A.; Gunel, M.K. Reliability of ashworth and modified ashworth scales in children with spastic cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.D.; Johnson, G.R.; Price, C.I.; Curless, R.H.; Barnes, M.P.; Rodgers, H. A review of the properties and limitations of the ashworth and modified ashworth scales as measures of spasticity. Clin. Rehabil. 1999, 13, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, J.; Grey, M.J.; Crone, C.; Mazevet, D.; Biering-Sørensen, F.; Nielsen, J.B. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin. Neurophysiol. 2010, 121, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Banky, M.; Ryan, H.K.; Clark, R.; Olver, J.; Williams, G. Do clinical tests of spasticity accurately reflect muscle function during walking: A systematic review. Brain Inj. 2017, 31, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Trompetto, C.; Mori, L.; Vigo, G.; Traverso, E.; Colombano, F.; Abbruzzese, G. Manual linear movements to assess spasticity in a clinical setting. PLoS ONE 2013, 8, e53627. [Google Scholar] [CrossRef][Green Version]
- Zurawski, E.; Behm, K.; Dunlap, C.; Koo, J.; Ismail, F.; Boulias, C.; Reid, S.; Phadke, C.P. Interrater reliability of the modified ashworth scale with standardized movement speeds: A pilot study. Physiother. Can. 2019, 71, 348–354. [Google Scholar] [CrossRef]
- Abolhasani, H.; Ansari, N.N.; Naghdi, S.; Mansouri, K.; Ghotbi, N.; Hasson, S. Comparing the validity of the modified modified ashworth scale (mmas) and the modified tardieu scale (mts) in the assessment of wrist flexor spasticity in patients with stroke: Protocol for a neurophysiological study. BMJ Open 2012, 2, e001394. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the sem. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Furlan, L.; Sterr, A. The applicability of standard error of measurement and minimal detectable change to motor learning research—A behavioral study. Front. Hum. Neurosci. 2018, 12, 95. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009; Volume 892. [Google Scholar]
- Meseguer-Henarejos, A.-B.; Sanchez-Meca, J.; Lopez-Pina, J.-A.; Carles-Hernandez, R. Inter-and intra-rater reliability of the modified ashworth scale: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017, 54, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.; Sinclair, E.; Thompson, J.; Taylor, S.; Pentland, B. Inter-rater reliability of the modified ashworth scale for spasticity in hemiplegic patients. Int. J. Rehabil. Res. 1992, 15, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Gregson, J.M.; Leathley, M.J.; Moore, A.P.; Smith, T.L.; Sharma, A.K.; Watkins, C.L. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 2000, 29, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.H.; Walt, S.E.; Lobb, G.; Stott, N.S. Intraobserver reliability of the modified tardieu scale in the upper limb of children with hemiplegia. Dev. Med. Child Neurol. 2004, 46, 267–272. [Google Scholar] [CrossRef] [PubMed]
| Rater 1 | Rater 2 | ||
|---|---|---|---|
| First measurement | Knee flexion | 131°/s (0.7%) | 136°/s (4.6%) |
| Knee extension | 124°/s (−4.6%) | 123°/s (−5.3%) | |
| Second measurement | Knee flexion | 132°/s (1.5%) | 136°/s (4.6%) |
| Knee extension | 127°/s (−2.3%) | 126°/s (−3.1%) |
| Muscle Group | First Measurement | Second Measurement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAS | MTS | R1 | R2 | R2-R1 | MAS | MTS | R1 | R2 | R2-R1 | |
| Ankle PF, Knee extended | 0.70 (0.55–0.80) | 0.68 (0.52–0.79) | 0.73 (0.59–0.82) | 0.83 (0.74–0.89) | 0.54 (0.30–0.70) | 0.73 (0.58–0.82) | 0.58 (0.34–0.72) | 0.80 (0.70–0.87) | 0.88 (0.82–0.92) | 0.65 (0.47–0.77) |
| Ankle PF, Knee flexed | 0.77 (0.65–0.84) | 0.82 (0.73–0.88) | 0.69 (0.51–0.80) | 0.88 (0.80–0.92) | 0.56 (0.34–0.71) | 0.82 (0.71–0.89) | 0.81 (0.71–0.87) | 0.68 (0.51–0.79) | 0.93 (0.88–0.95) | 0.66 (0.48–0.77) |
| Muscle Group | Rater 1 | Rater 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAS | MTS | R1 | R2 | R2-R1 | MAS | MTS | R1 | R2 | R2-R1 | |
| Ankle PF, Knee extended | 0.76 (0.64–0.84) | 0.70 (0.54–0.80) | 0.84 (0.76–0.90) | 0.74 (0.60–0.83) | 0.68 (0.51–0.79) | 0.83 (0.74–0.89) | 0.74 (0.61–0.83) | 0.78 (0.67–0.85) | 0.84 (0.76–0.89) | 0.62 (0.42–0.75) |
| Ankle PF, Knee flexed | 0.83 (0.75–0.89) | 0.76 (0.63–0.84) | 0.76 (0.64–0.84) | 0.84 (0.76–0.90) | 0.71 (0.57–0.81) | 0.88 (0.82–0.92) | 0.78 (0.67–0.85) | 0.82 (0.73–0.88) | 0.90 (0.84–0.93) | 0.74 (0.61–0.83) |
| MAS | MTS | R1 | R2 | R2-R1 | |
|---|---|---|---|---|---|
| Ankle PF, Knee extended | 0.31 | 0.35 | 4.71 | 4.25 | 6.18 |
| Ankle PF, Knee flexed | 0.32 | 0.33 | 6.53 | 3.80 | 6.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, M.; Ahn, J.H.; Rha, D.-w.; Park, E.S. Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children 2022, 9, 827. https://doi.org/10.3390/children9060827
Yoo M, Ahn JH, Rha D-w, Park ES. Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children. 2022; 9(6):827. https://doi.org/10.3390/children9060827
Chicago/Turabian StyleYoo, Myungeun, Jeong Hyeon Ahn, Dong-wook Rha, and Eun Sook Park. 2022. "Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy" Children 9, no. 6: 827. https://doi.org/10.3390/children9060827
APA StyleYoo, M., Ahn, J. H., Rha, D.-w., & Park, E. S. (2022). Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children, 9(6), 827. https://doi.org/10.3390/children9060827






