The Performance Comparison of Socioeconomic and Behavioural Factors as Predictors of Higher Blood Lead Levels of 0–6-Year-Old Chinese Children between 2004 and 2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaire
2.3. Blood Lead Concentration Laboratory Tests
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavaghan, H. Lead, unsafe at any level. Bull. World Health Organ. 2002, 80, 82. [Google Scholar] [PubMed]
- He, J.; Ning, H.; Huang, R. Low blood lead levels and attention-deficit hyperactivity disorder in children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2017, 26, 17875–17884. [Google Scholar] [CrossRef] [PubMed]
- Jusko, T.A.; Henderson, C.R.; Lanphear, B.P.; Cory-Slechta, D.A.; Parsons, P.J.; Canfield, R.L. Blood lead concentrations <10 microg/dL and child intelligence at 6 years of age. Environ. Health Perspect. 2008, 116, 243–248. [Google Scholar] [CrossRef]
- Binns, H.J.; Campbell, C.; Brown, M.J. Interpreting and managing blood lead levels of less than 10 microg/dL in children and reducing childhood exposure to lead: Recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics 2007, 120, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dai, Y.H.; Xie, X.H.; Tan, Z.W.; Zhang, S.M.; Zhu, Z.H. Surveillance of childhood blood lead levels in 11 cities of China. World J. Pediatr. WJP 2014, 10, 29–37. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Tan, Z.; Dai, Y. Trend of childhood blood lead levels in cities of China in recent 10 years. Environ. Sci. Pollut. Res. Int. 2017, 24, 5824–5830. [Google Scholar] [CrossRef]
- Barbosa, F., Jr.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- Etchevers, A.; Bretin, P.; Lecoffre, C.; Bidondo, M.L.; Le Strat, Y.; Glorennec, P.; Le Tertre, A. Blood lead levels and risk factors in young children in France, 2008–2009. Int. J. Hyg. Environ. Health 2014, 217, 528–537. [Google Scholar] [CrossRef]
- Kaplowitz, S.A.; Perlstadt, H.; D’Onofrio, G.; Melnick, E.R.; Baum, C.R.; Kirrane, B.M.; Post, L.A. The predictive value of self-report questions in a clinical decision rule for pediatric lead poisoning screening. Public Health Rep. 2012, 127, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Gleason, J.; Nanavaty, J.; Fagliano, J. Drinking water lead and socioeconomic factors as predictors of blood lead levels in New Jersey’s children between two time periods. Environ. Res. 2018, 169, 409–416. [Google Scholar] [CrossRef]
- Levin, R.; Brown, M.J.; Kashtock, M.E.; Jacobs, D.E.; Whelan, E.A.; Rodman, J.; Schock, M.R.; Padilla, A.; Sinks, T. Lead exposures in U.S. Children, 2008: Implications for prevention. Environ. Health Perspect. 2008, 116, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.Z.; Wu, F.; Yan, C.H.; Li, K.; Liu, X.Y. Childhood lead poisoning associated with traditional Chinese medicine: A case report and the subsequent lead source inquiry. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Xu, J.; Shen, X.M. Childhood lead poisoning in China: Challenges and opportunities. Environ. Health Perspect. 2013, 121, A294–A295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomchai, C.; Padungtod, C.; Chomchai, S. Predictors of elevated blood lead level in Thai children: A pilot study using risk assessment questionnaire. J. Med. Assoc. Thai. 2005, 88, 53–59. [Google Scholar]
- Bolte, G.; Tamburlini, G.; Kohlhuber, M. Environmental inequalities among children in Europe—Evaluation of scientific evidence and policy implications. Eur. J. Public Health 2010, 20, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Ha, M.; Hwang, S.S.; Son, M.; Kwon, H.J. Disparities in Children’s Blood Lead and Mercury Levels According to Community and Individual Socioeconomic Positions. Int. J. Environ. Res. Public Health 2015, 12, 6232–6248. [Google Scholar] [CrossRef] [Green Version]
- McClure, L.F.; Niles, J.K.; Kaufman, H.W. Blood Lead Levels in Young Children: US, 2009–2015. J. Pediatr. 2016, 175, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Li, M.M.; Cao, J.; Gao, Z.Y.; Shen, X.M.; Yan, C.H. The trend of lead poisoning rate in Chinese population aged 0–18 years old: A meta-analysis. BMC Public Health 2015, 15, 756. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Wang, S.; Zhang, J. Blood lead levels of children and its trend in China. Sci. Total Environ. 2009, 407, 3986–3993. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- Peng, T.; O’Connor, D.; Zhao, B.; Jin, Y.; Zhang, Y.; Tian, L.; Zheng, N.; Li, X.; Hou, D. Spatial distribution of lead contamination in soil and equipment dust at children’s playgrounds in Beijing, China. Environ. Pollut. 2019, 245, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Edwards, R.; He, X.E.; Liu, Z.; Kleinman, M. Spatial analysis of bioavailable soil lead concentrations in Los Angeles, California. Environ. Res. 2010, 110, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R. Lead-Based Decorative Paints: Where Are They Still Sold-and Why? Environ. Health Perspect. 2014, 122, A96–A103. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.S.; Rampal, K.G.; Thuppil, V.; Chen, C.K.; Clark, R.; Roda, S. The lead content of currently available new residential paint in several Asian countries. Environ. Res. 2006, 102, 9–12. [Google Scholar] [CrossRef]
- Lin, G.Z.; Peng, R.F.; Chen, Q.; Wu, Z.G.; Du, L. Lead in housing paints: An exposure source still not taken seriously for children lead poisoning in China. Environ. Res. 2009, 109, 1–5. [Google Scholar] [CrossRef]
- Chiaradia, M.; Gulson, B.L.; MacDonald, K. Contamination of houses by workers occupationally exposed in a lead-zinc-copper mine and impact on blood lead concentrations in the families. Occup. Environ. Med. 1997, 54, 117–124. [Google Scholar] [CrossRef]
- Hauptman, M.; Bruccoleri, R.; Woolf, A.D. An Update on Childhood Lead Poisoning. Clin. Pediatr. Emerg. Med. 2017, 18, 181–192. [Google Scholar] [CrossRef]
- Shen, Z.; Hou, D.; Zhang, P.; Wang, Y.; Zhang, Y.; Shi, P.; O’Connor, D. Lead-based paint in children’s toys sold on China’s major online shopping platforms. Environ. Pollut. 2018, 241, 311–318. [Google Scholar] [CrossRef]
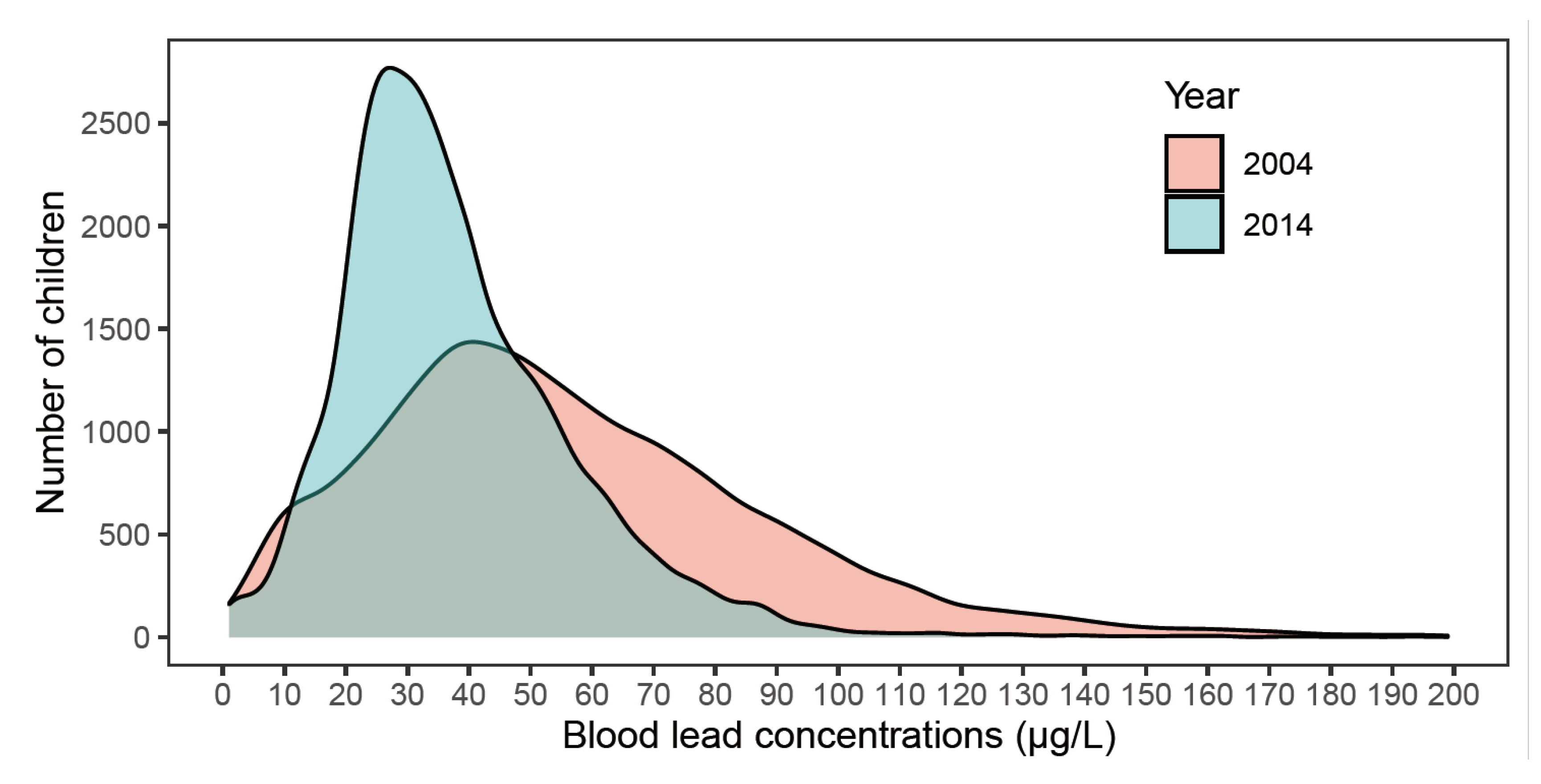
| 2004 | 2014 | p Value # | |
|---|---|---|---|
| N (%) | N (%) | ||
| Total | 13,852 (100) | 14,120 (100) | |
| Sex | |||
| Female | 6173 (44.56) | 6442 (45.62) | 0.0799 |
| Age | <0.0001 | ||
| 0– | 1316 (9.50) | 1366 (9.67) | |
| 1– | 1083 (7.82) | 1397 (9.89) | |
| 2– | 1686 (12.17) | 1363 (9.65) | |
| 3– | 3092 (22.32) | 2679 (18.97) | |
| 4– | 2958 (21.35) | 3181 (22.53) | |
| 5– | 2662 (19.22) | 2723 (19.28) | |
| 6–7 | 1055 (7.62) | 1411 (9.99) | |
| Father’s education | <0.0001 | ||
| College graduate or beyond | 8026 (58.59) | 9574 (68.02) | |
| High school graduate | 4106 (29.98) | 3327 (23.63) | |
| Junior high school or lower | 1566 (11.43) | 1175 (8.35) | |
| Blood lead concentration (GM (95% CI), μg/L) | 46.76 (46.21, 47.33) | 33.37 (33.05, 33.70) | <0.0001 * |
| Percentage ≥ 50 μg/L (%) | 52.53 | 22.90 | <0.0001 |
| Percentage ≥ 100 μg/L (%) | 10.17 | 1.14 | <0.0001 |
| Factors | 2004 | 2014 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| % | GM (95% CI) | Change, % | GM (95% CI) | Change, % | % | GM (95% CI) | Change, % | GM (95% CI) | Change, % | |
| Sex | ||||||||||
| Female | 44.59 | 44.73 (43.93, 45.54) a | - | 47.96 (45.97, 50.03) a | - | 45.63 | 33.54 (33.07, 34.02) a | -. | 42.71 (40.87, 44.64) a | - |
| Male | 55.41 | 48.45 (47.68, 49.23) b | 8.32 | 51.78 (49.69, 53.97) b | 7.96 | 54.37 | 33.23 (32.79, 33.67) a | −0.92 | 42.31 (40.52, 44.19) a | −0.94 |
| Age | ||||||||||
| 0– | 9.50 | 37.52 (36.00, 39.11) a | - | 41.46 (39.13, 43.92) a | 9.67 | 30.22 (29.10, 31.39) a | - | 38.31 (36.28, 40.46) a | ||
| 1– | 7.82 | 44.85 (43.19, 46.57) b | 19.54 | 47.83 (45.09, 50.72) b | 15.36 | 9.89 | 31.69 (30.60, 32.83) a | 4.86 | 40.18 (38.13, 42.35) a | 4.88 |
| 2– | 12.17 | 46.07 (44.59, 47.59) c | 22.79 | 48.39 (45.96, 50.95) b | 16.71 | 9.65 | 31.81 (30.80, 32.86) a | 5.26 | 40.70 (38.62, 42.90) a | 6.24 |
| 3– | 22.32 | 46.68 (45.51, 47.88) c | 24.41 | 49.85 (47.60, 52.21) b | 20.24 | 18.97 | 33.89 (33.20, 34.61) a | 12.14 | 43.99 (41.99, 46.07) b | 14.83 |
| 4– | 21.35 | 46.75 (45.54, 48.00) c | 24.6 | 49.40 (47.20, 51.70) b | 19.15 | 22.53 | 34.40 (33.80, 35.01) b | 13.83 | 45.15 (43.12, 47.28) b | 17.85 |
| 5– | 19.22 | 49.73 (48.35, 51.15) d | 32.54 | 53.46 (51.03, 56.01) c | 28.94 | 19.28 | 34.00 (33.27, 34.74) b | 12.51 | 44.52 (42.50, 46.64) b | 16.21 |
| 6–7 | 7.62 | 56.65 (54.59, 58.79) e | 50.99 | 60.42 (56.98, 64.06) d | 45.73 | 9.99 | 35.35 (34.28, 36.46) c | 16.98 | 45.31 (43.11, 47.62) b | 18.27 |
| Father’s education | ||||||||||
| College graduate or beyond | 58.59 | 45.32 (44.62, 46.04) a | - | 48.09 (46.07, 50.20) a | 68.02 | 32.24 (31.87, 32.62) a | - | 40.04 (38.32, 41.84) a | ||
| High school graduate | 29.98 | 48.54 (47.47, 49.64) b | 7.11 | 50.57 (48.43, 52.80) b | 5.16 | 23.64 | 34.70 (34.03, 35.38) b | 7.63 | 41.49 (39.67, 43.40) b | 3.62 |
| Junior high school or lower | 11.43 | 49.36 (47.69, 51.10) b | 8.91 | 50.89 (48.36, 53.55) b | 5.82 | 8.35 | 39.38 (37.98, 40.85) c | 22.15 | 46.26 (43.93, 48.71) c | 15.53 |
| Distance from residence to main road | ||||||||||
| Far from the main road | 35.89 | 46.24 (45.31, 47.19) a | - | 49.36 (47.24, 51.58) a | 38.78 | 31.40 (30.86, 31.94) a | - | 39.30 (37.56, 41.13) a | ||
| Separated by 2 buildings | 17.84 | 45.75 (44.48, 47.05) a | −1.06 | 49.41 (47.09, 51.85) a | 0.1 | 15.63 | 33.66 (32.91, 34.42) b | 7.2 | 42.09 (40.10, 44.17) b | 7.1 |
| Separated by 1 building | 14.90 | 46.06 (44.57, 47.59) a | −0.39 | 48.88 (46.52, 51.36) a | −0.97 | 19.77 | 34.46 (33.80, 35.13) bc | 9.75 | 43.59 (41.60, 45.67) b | 10.92 |
| Separated by roadside green belt | 13.68 | 47.06 (45.55, 48.61) a | 1.77 | 50.60 (48.10, 53.23) a | 2.51 | 12.22 | 35.38 (34.52, 36.27) c | 12.68 | 44.68 (42.52, 46.95) c | 13.69 |
| Facing the street | 17.69 | 47.91 (46.57, 49.29) a | 3.61 | 50.94 (48.62, 53.38) a | 3.2 | 13.60 | 35.60 (34.66, 36.57) c | 13.38 | 43.12 (41.10, 45.24) b | 9.72 |
| Peeling-off wall and paint in the room | ||||||||||
| No | 80.53 | 46.70 (46.08, 47.32) a | - | 50.43 (48.34, 52.60) a | 84.20 | 32.97 (32.62, 33.32) a | - | 42.09 (40.28, 43.99) a | ||
| Yes | 19.47 | 46.68 (45.35, 48.04) a | −0.04 | 49.24 (47.11, 51.48) a | −2.36 | 15.80 | 35.61 (34.80, 36.44) b | 8.01 | 42.94 (41.02, 44.96) a | 2.02 |
| Peeling-off furniture paint in the room | ||||||||||
| No | 92.40 | 46.45 (45.86, 47.05) a | - | 49.59 (47.71, 51.54) a | 89.99 | 33.13 (32.8, 33.47) a | - | 41.72 (40.01, 43.51) a | ||
| Yes | 7.60 | 47.79 (45.95, 49.70) a | 2.88 | 50.08 (47.55, 52.73) a | 0.99 | 10.01 | 36.49 (35.2, 37.82) b | 10.14 | 43.32 (41.15, 45.61) a | 3.84 |
| Having Chinese traditional medicine | ||||||||||
| Less than 2 times per year | 66.43 | 46.20 (45.51, 46.90) a | - | 49.01 (47.08, 51.03) a | 78.61 | 32.66 (32.30, 33.02) a | - | 39.92 (38.27, 41.64) a | ||
| 1−2 times per month | 25.88 | 47.62 (46.52, 48.75) a | 3.07 | 49.59 (47.49, 51.78) a | 1.18 | 15.97 | 34.94 (34.14, 35.77) b | 6.98 | 41.28 (39.41, 43.24) b | 3.41 |
| More than once per week | 7.70 | 48.55 (46.59, 50.58) a | 5.09 | 50.92 (48.09, 53.91) a | 2.68 | 5.41 | 39.61 (38.00, 41.29) c | 21.28 | 46.63 (44.07, 49.34) c | 16.81 |
| Father’s occupation having contact with lead | ||||||||||
| No | 82.73 | 46.24 (45.64, 46.86) b | - | 49.29 (47.24, 51.42) a | 87.24 | 32.59 (32.25, 32.93) a | - | 40.01 (38.18, 41.93) a | ||
| Yes | 17.27 | 48.94 (47.52, 50.40) a | 5.84 | 50.38 (48.17, 52.70) a | 2.21 | 12.76 | 39.17 (38.18, 40.20) b | 20.19 | 45.17 (43.19, 47.25) b | 12.9 |
| Mother’s occupation having contact with lead | ||||||||||
| No | 94.52 | 46.57 (46,00, 47.15) a | - | 49.33 (47.66, 51.05) a | 96.37 | 33.15 (32.82, 33.48) a | - | 41.81 (40.27, 43.41) a | ||
| Yes | 5.48 | 48.95 (46.37, 51.68) a | 5.11 | 50.34 (47.42, 53.45) a | 2.05 | 3.63 | 39.61 (37.92, 41.38) b | 19.49 | 43.23 (40.62, 46.02) a | 3.4 |
| Whether children often suck fingers | ||||||||||
| No | 69.23 | 46.74 (46.07, 47.43) a | - | 49.68 (47.65, 51.79) a | 72.35 | 32.99 (32.63, 33.35) a | - | 41.60 (39.79, 43.50) a | ||
| Yes | 30.77 | 46.53 (45.54, 47.55) a | −0.45 | 49.99 (47.89, 52.18) a | 0.62 | 27.65 | 34.40 (33.71, 35.10) b | 4.27 | 43.45 (41.58, 45.40) b | 4.45 |
| Pica | ||||||||||
| No | 86.01 | 46.21 (45.61, 46.81) b | - | 49.42 (47.50, 51.42) a | 96.64 | 33.25 (32.93, 33.58) a | - | 41.51 (40.05, 43.02) a | ||
| Yes | 13.99 | 49.77 (48.23, 51.36) a | 7.7 | 50.24 (47.88, 52.72) a | 1.66 | 3.36 | 37.16 (35.34, 39.06) b | 11.76 | 43.54 (40.93, 46.32) a | 4.89 |
| Whether children often bite stationery | ||||||||||
| No | 65.65 | 45.28 (44.62, 45.95) b | - | 49.38 (47.25, 51.60) a | 91.25 | 33.07 (32.74, 33.41) a | - | 42.00 (40.25, 43.82) a | ||
| Yes | 34.35 | 49.64 (48.61, 50.69) a | 9.63 | 50.29 (48.28, 52.39) a | 1.84 | 8.75 | 36.77 (35.61, 37.97) b | 11.19 | 43.04 (40.94, 45.24) a | 2.48 |
| Whether children often bite toys | ||||||||||
| No | 89.76 | 45.96 (45.38, 46.55) b | - | 47.99 (46.18, 49.88) a | 83.02 | 32.83 (32.48, 33.18) a | - | 40.93 (39.15, 42.79) a | ||
| Yes | 10.24 | 53.45 (51.57, 55.40) a | 16.3 | 51.74 (49.12, 54.51) b | 7.81 | 16.98 | 36.18 (35.35, 37.03) b | 10.2 | 44.16 (42.20, 46.21) b | 7.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Dai, Y.; Li, T. The Performance Comparison of Socioeconomic and Behavioural Factors as Predictors of Higher Blood Lead Levels of 0–6-Year-Old Chinese Children between 2004 and 2014. Children 2022, 9, 802. https://doi.org/10.3390/children9060802
Xie Y, Dai Y, Li T. The Performance Comparison of Socioeconomic and Behavioural Factors as Predictors of Higher Blood Lead Levels of 0–6-Year-Old Chinese Children between 2004 and 2014. Children. 2022; 9(6):802. https://doi.org/10.3390/children9060802
Chicago/Turabian StyleXie, Yixuan, Yaohua Dai, and Tao Li. 2022. "The Performance Comparison of Socioeconomic and Behavioural Factors as Predictors of Higher Blood Lead Levels of 0–6-Year-Old Chinese Children between 2004 and 2014" Children 9, no. 6: 802. https://doi.org/10.3390/children9060802
APA StyleXie, Y., Dai, Y., & Li, T. (2022). The Performance Comparison of Socioeconomic and Behavioural Factors as Predictors of Higher Blood Lead Levels of 0–6-Year-Old Chinese Children between 2004 and 2014. Children, 9(6), 802. https://doi.org/10.3390/children9060802






