Three-Dimensional versus Two-Dimensional Evaluations of Cranial Asymmetry in Deformational Plagiocephaly Using a Three-Dimensional Scanner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Data Acquisition Using the 3D Scanner
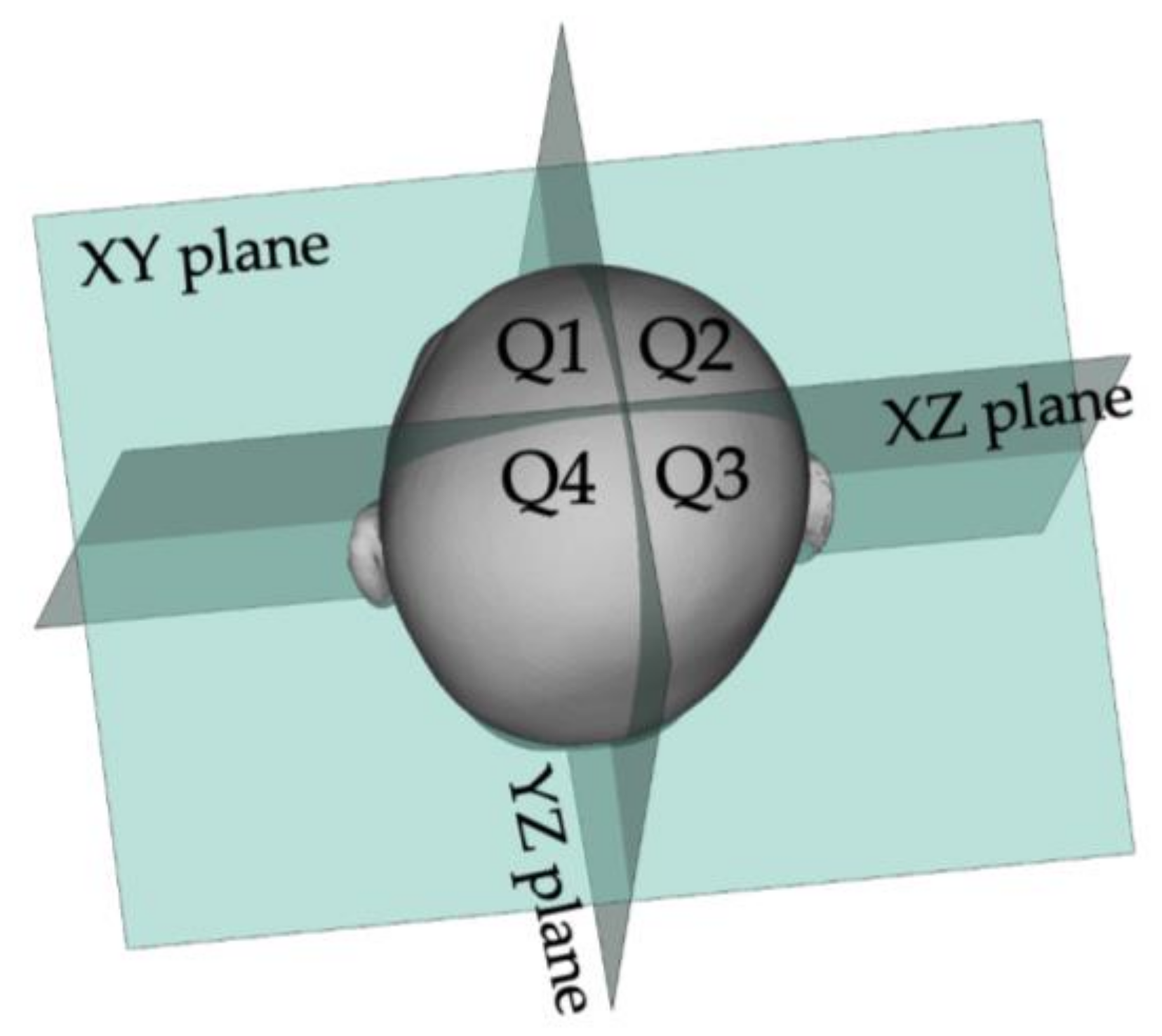
2.3. Data Analysis Method
2.3.1. How to Calculate ASR and PSR
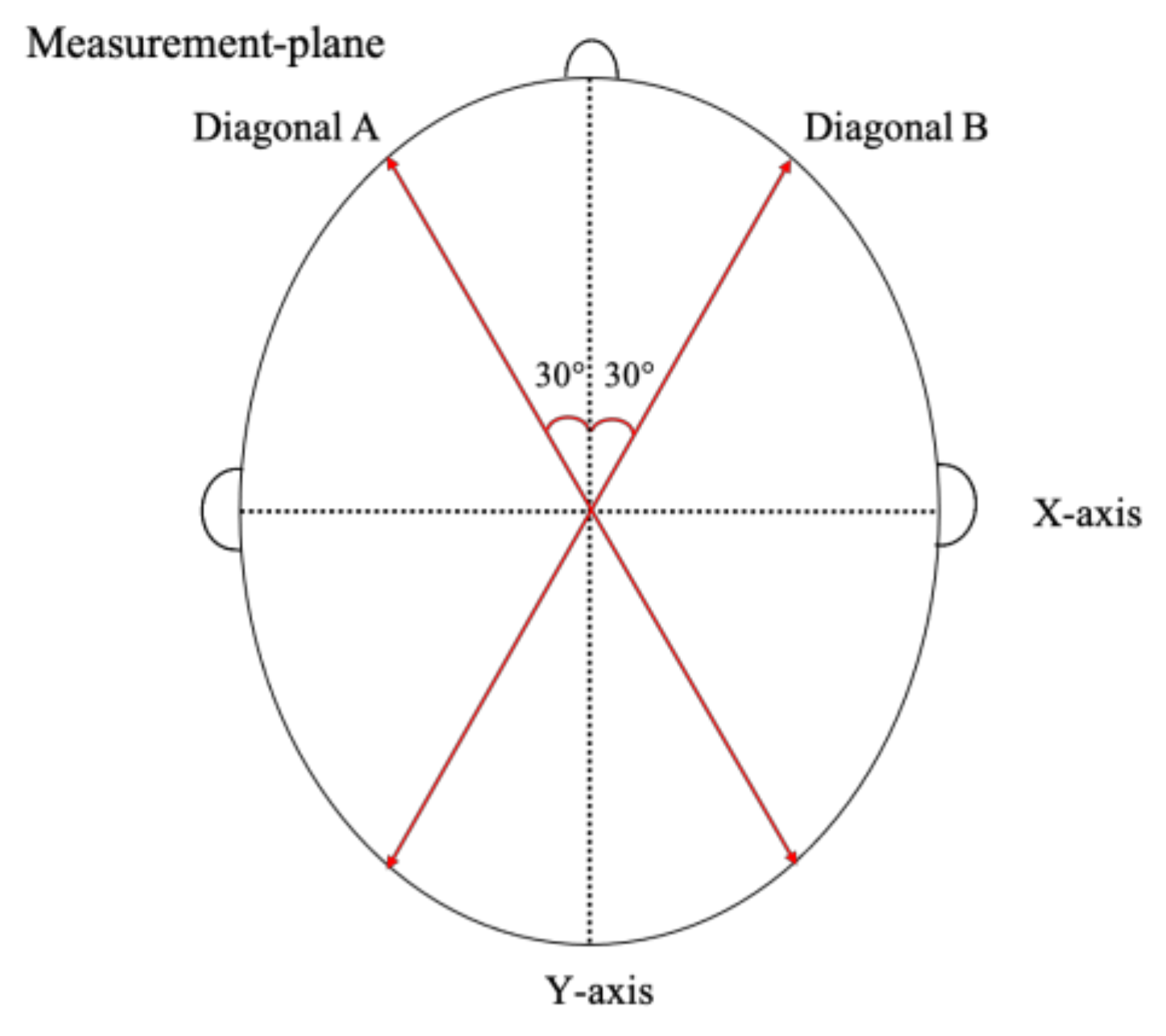
2.3.2. Cross-Sectional Level for 2D Measurement
2.3.3. How to Calculate CA
2.4. Severity Classifications
2.5. Study Methods and Statistical Analyses
- (1)
- Age, height, weight, and head circumference at the measurement date were collected.
- (2)
- To determine the repeatability of the 3D scanner, six replicates of the scans were performed by one examiner (intra-examiner precision analysis). Six replicate scans were then performed by six different examiners (one scan per examiner; inter-examiner precision analysis). Mean, standard deviation (SD), and coefficient of variation (CV) were then calculated.
- (1)
- Perinatal and neonatal factors included sex, gestational weeks at birth, birth weight, age at the time of measurement, mode of delivery, and intrauterine position.
- (2)
- The values of head circumferences measured by the 3D scanner and by trained nurses were compared using bivariable normal ellipses, and regression analysis (correlation coefficient [r] was calculated).
- (3)
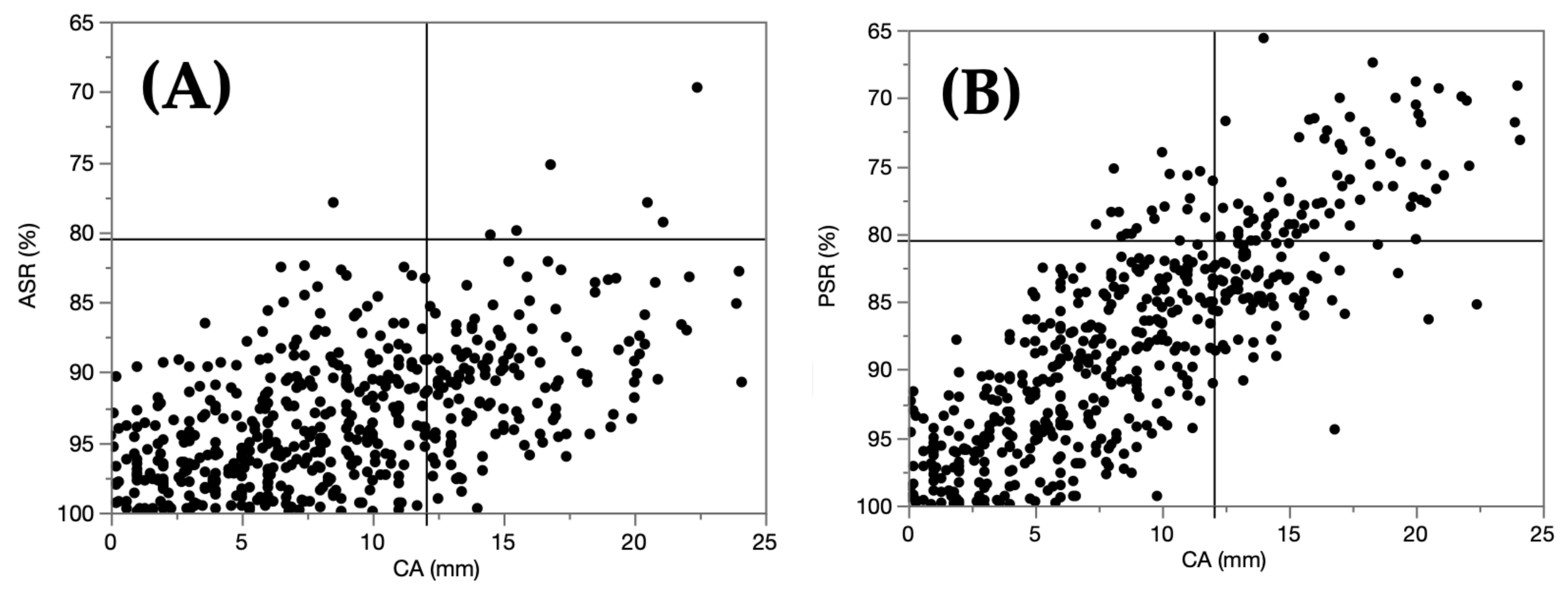
- Distribution maps of the ASRs, PSRs, and CAs are shown. Each infant was classified by severity (mild or severe) using CA for the 2D evaluation, and ASR alone, PSR alone, or ASR and PSR for the 3D evaluation. The coincidence rate was analyzed, and the indicative variable in the 3D evaluation that showed the maximum coincidence rate was determined.
- (4)
- Finally, infants whose severity assessed differently, especially the group identified as mild on the 2D evaluation and severe on the 3D evaluation, were selected. Using CA on planes other than level 3 at levels 2–8, we investigated whether there was a change in the coincidence rate.
3. Results
3.1. Study 1
3.1.1. Subject
3.1.2. Measurement Precision
3.2. Study 2
3.2.1. Clinical Characteristics
3.2.2. Correlation of Measurement Values
3.2.3. Severity Classifications
3.2.4. CAs of Other Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Looman, W.S.; Flannery, A.B. Evidence-based care of the child with deformational plagiocephaly, Part I: Evaluation and diagnosis. J. Pediatr. Health Care 2012, 26, 250. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Marcotty, P.; Kunz, F.; Schweitzer, T.; Wachter, B.; Böhm, H.; Waßmuth, N.; Linz, C. Cranial growth in infants—A longitudinal three-dimensional analysis of the first months of life. J. Craniomaxillofac. Surg. 2018, 46, 987–993. [Google Scholar] [CrossRef] [PubMed]
- González-Santos, J.; González-Bernal, J.J.; De-la-Fuente Anuncibay, R.; Soto-Cámara, R.; Cubo, E.; Aguilar-Parra, J.M.; Trigueros, R.; López-Liria, R. Infant Cranial Deformity: Cranial Helmet Therapy or Physiotherapy? Int. J. Environ. Res. Public Health 2020, 17, 2612. [Google Scholar] [CrossRef]
- Meyer-Marcotty, P.; Böhm, H.; Linz, C.; Kochel, J.; Stellzig-Eisenhauer, A.; Schweitzer, T. Three-dimensional analysis of cranial growth from 6 to 12 months of age. Eur. J. Orthod. 2014, 36, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Pons, I.; Lucha-López, M.O.; Barrau-Lalmolda, M.; Rodes-Pastor, I.; Rodríguez-Fernández, Á.L.; Hidalgo-García, C. Tricás-Moreno, J.M. Interratrer and Intrarater Reliability of Cranial Anthropometric Measurements in Infants with Deformational plagiocephaly. Children 2020, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, F.; Ble, V.; Beuriat, P.A.; Szathmari, A.; Lohkamp, L.N.; Mottolese, C. Prevalence and severity of deformational plagiocephaly in children and adolescents. Acta Neurochir. 2019, 161, 1095–1098. [Google Scholar] [CrossRef]
- Mortenson, P.A.; Steinbok, P. Quantifying deformational plagiocephaly: Reliability and validity of anthropometric measurements. J. Craniofac. Surg. 2006, 17, 413–419. [Google Scholar] [CrossRef]
- Schaaf, H.; Wilbrand, J. Accuracy of photographic evaluation compared with standard anthropometric measurements in nonsynostotic cranial deformities. Cleft Palate Craniofac. J. 2010, 47, 447–453. [Google Scholar] [CrossRef]
- Lipira, A.B.; Gordon, S.; Darvann, T.A.; Hermann, N.V.; Van Pelt, A.E.; Naidoo, S.D.; Govier, D.; Kane, A.A. Helmet versus active repositioning for plagiocephaly: A three-dimensional analysis. Pediatrics 2010, 126, e936–e945. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, M.S.; Yang, J.I.; Yim, S.Y. Comparison of helmet therapy and counter positioning for deformational plagiocephaly. Ann. Rehabil. Med. 2013, 37, 785–795. [Google Scholar] [CrossRef]
- Aihara, Y.; Komatsu, K.; Dairoku, H.; Kubo, O.; Hori, T.; Okada, Y. Cranial molding helmet therapy and establishment of practical criteria for management in Asian Infant positional head deformity. Child’s Nerv. Syst. 2014, 30, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Noto, T.; Nagano, N.; Kato, R.; Hashimoto, S.; Saito, K.; Miyabayashi, H.; Sasano, M.; Sumi, K.; Yoshino, A.; Morioka, I. Natural-course evaluation of infants with positional severe plagiocephaly using a three-dimensional scanner in Japan: Comparison with those who received cranial helmet therapy. J. Clin. Med. 2021, 10, 3531. [Google Scholar] [CrossRef] [PubMed]
- Lazovic, G.D.; Daniel, R.K.; Janosevic, L.B.; Kosanovic, R.M.; Colic, M.M.; Kosins, A.M. Rhinoplasty: The nasal bones—Anatomy and analysis. Aesthet Surg J. 2015, 35, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bahşi, I.; Orhan, M.; Kervancioğlu, P. The confusion of determining the landmark in the craniofacial soft tissue analyzes: Nasion or sellion? J. Craniofac. Surg. 2021, 32, e602–e603. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M.Y.; Moerenhout, B.A. Reliability of clinical measurements used in the determination of facial indices. J. Craniomaxillofac. Surg. 2008, 36, 279–284. [Google Scholar] [CrossRef]
- Plank, L.H.; Giavedoni, B.; Lombardo, J.R.; Geil, M.D.; Reisner, A. Comparison of infant head shape changes in deformational plagiocephaly following treatment with a cranial remolding orthosis using a nonivasive laser shape digitizer. J. Craniofac. Surg. 2006, 17, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Ifflaender, S.; Rüdiger, M.; Koch, A.; Burkhardt, W. Three-dimensional digital capture of head size in neonates—A method evaluation. PLoS ONE 2013, 8, e61274. [Google Scholar] [CrossRef] [Green Version]
- Kunz, F.; Schweitzer, T.; Große, S.; Waßmuth, N.; Stellzig-Eisenhauer, A.; Böhm, H.; Meyer-Marcotty, P.; Linz, C. Head orthosis therapy in deformational plagiocephaly: Longitudinal 3D-investigation of long-term outcomes, compared with untreated infants and with a control group. Eur. J. Orthod. 2019, 41, 29–37. [Google Scholar] [CrossRef]
- Loveday, B.P.; de Chalain, T.B. Active counterpositioning or orthotic device to treat deformational plagiocephaly? J. Craniofac. Surg. 2001, 12, 308–313. [Google Scholar] [CrossRef]
- Mortenson, P.; Steinbok, P.; Smith, D. Deformational plagiocephaly and orthotic treatment: Indications and limitations. Childs Nerv. Syst. 2012, 28, 1407–1412. [Google Scholar] [CrossRef]
- Fahrenkopf, M.P.; Adams, N.S.; Mann, R.J.; Girotto, J.A. Deformational plagiocephaly. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St Geme, J.W., Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 3082–3086. [Google Scholar]
- Branch, L.G.; Kesty, K.; Krebs, E.; Wright, L.; Leger, S.; David, L.R. Deformational plagiocephaly and craniosynostosis: Trends in diagnosis and treatment after the "back to sleep" campaign. J. Craniofac. Surg. 2015, 26, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.; David, L.; Thompson, J. Clinical classification of positional plagiocephaly. J. Craniofac. Surg. 2004, 15, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Aarnivala, H.; Vuollo, V.; Heikkinen, T.; Harila, V.; Holmström, L.; Pirttiniemi, P.; Valkama, A.M. Accuracy of measurements used to quantify cranial asymmetry in deformational plagiocephaly. J. Craniomaxillofac. Surg. 2017, 45, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Verhulst, A.; Hol, M.; Vreeken, R.; Becking, A.; Ulrich, D.; Maal, T. Three-dimensional imaging of the face: A comparison between three different imaging modalities. Aesthet. Surg. J. 2018, 38, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyabayashi, H.; Nagano, N.; Kato, R.; Noto, T.; Hashimoto, S.; Saito, K.; Morioka, I. Reference values for cranial morphology based on three-dimensional scan analysis in 1-month-old healthy infants in Japan. Neurol. Med. Chir. 2022, 62, 246–253. [Google Scholar] [CrossRef]


| Intra-Examiner | Inter-Examiner | |||
|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| n = 6 | n = 6 | |||
| Cranial length, mm | 202.3 ± 0.5 | 0.26 | 203.0 ± 0.8 | 0.39 |
| Cranial width, mm | 171.7 ± 0.2 | 0.13 | 171.8 ± 0.3 | 0.16 |
| Head circumference, mm | 582.9 ± 1.2 | 0.20 | 582.9 ± 0.7 | 0.12 |
| CA, mm | 10.0 ± 0.6 | 5.62 | 10.0 ± 0.5 | 5.33 |
| ASR, % | 98.7 ± 0.2 | 0.24 | 98.6 ± 0.2 | 0.15 |
| PSR, % | 83.4 ± 0.4 | 0.50 | 83.7 ± 0.3 | 0.38 |
| Male, % | 315 (59.4) |
| Gestational age at birth, weeks | 39 (37–42) |
| Birth weight, grams | 3024 (1639–4144) |
| Age at measurement, months | 3 (0–47) |
| Mode of delivery | |
| Vaginal | 313 (59.0) |
| Caesarean | 161 (30.3) |
| Vacuum | 45 (8.4) |
| Forceps | 11 (2.0) |
| Intrauterine position | |
| Cephalic | 494 (93.2) |
| Breech | 31 (5.8) |
| Transverse | 5 (0.9) |
| (A) | CA | |||
|---|---|---|---|---|
| Mild | Severe | Total | ||
| ASR ≥ 80.5% | Mild | 383 (72.3%) | 140 (26.4%) | 523 (98.7%) |
| ASR < 80.5% | Severe | 1 (0.2%) | 6 (1.1%) | 7 (1.3%) |
| Total | 384 (72.5 %) | 146 (27.5 %) | 530 | |
| The severity coincidence rate was 73.4% (389/530). | ||||
| (B) | Mild | Severe | Total | |
| PSR ≥ 80.5% | Mild | 365 (68.9%) | 73 (13.8%) | 438 (82.6%) |
| PSR < 80.5% | Severe | 19 (3.6%) | 73 (13.8%) | 92 (17.4%) |
| Total | 384 (72.5%) | 146 (27.5%) | 530 | |
| The severity coincidence rate was 82.6% (438/530). | ||||
| (C) | Mild | Severe | Total | |
| ASR and PSR ≥ 80.5% | Mild | 364 (68.7%) | 68 (12.8%) | 432 (81.5%) |
| ASR or PSR < 80.5% | Severe | 20 (3.8%) | 78 (14.7%) | 98 (18.5%) |
| Total | 384 (72.5%) | 146 (27.5%) | 530 | |
| The severity coincidence rate was 83.4% (442/530). | ||||
| No. | CA (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Level 2 | Level 3 (Standard) | Level 4 | Level 5 | Level 6 | Level 7 | Level 8 | |
| 1 | 8 | 9.0 | 8.3 | 8.9 | 9.4 | 8.2 | 5.5 |
| 2 | 10.9 | 11.5 | 13.9 | 15.9 | 15.7 | 13.7 | 10.9 |
| 3 | 9.4 | 9.6 | 9.8 | 10.9 | 10.7 | 9.4 | 7.7 |
| 4 | 7 | 8.8 | 8.2 | 8.6 | 9.4 | 10.0 | 8.6 |
| 5 | 8.3 | 8.4 | 9.4 | 11.5 | 11.8 | 10.3 | 7.4 |
| 6 | 8.9 | 10.1 | 11.5 | 12.5 | 12.2 | 11.3 | 8.8 |
| 7 | 9.7 | 11.1 | 11.1 | 11.2 | 10.9 | 9.5 | 7.9 |
| 8 | 10.7 | 11.7 | 12.3 | 12.6 | 11.8 | 10.1 | 8.4 |
| 9 | 12.2 | 10.3 | 14.2 | 15.7 | 15.6 | 13.4 | 8.8 |
| 10 | 6.2 | 10.0 | 12.2 | 14.7 | 13.6 | 11.7 | 9.1 |
| 11 | 5.5 | 7.4 | 6.3 | 8.0 | 9.4 | 9.1 | 7.4 |
| 12 | 7.3 | 8.3 | 9.1 | 9.4 | 9.6 | 7.8 | 6.4 |
| 13 | 8.5 | 8.5 | 10.1 | 9.6 | 8.6 | 7.2 | 5.6 |
| 14 | 7.1 | 9.7 | 7.8 | 10.3 | 12.2 | 11.9 | 10.7 |
| 15 | 8.8 | 12.0 | 16.9 | 18.7 | 17.7 | 15.3 | 11.7 |
| 16 | 10.2 | 11.0 | 11.1 | 11.3 | 11.1 | 10.3 | 8.5 |
| 17 | 8.5 | 11.0 | 10.7 | 12.4 | 12.1 | 10.5 | 9 |
| 18 | 6.5 | 8.0 | 9.8 | 10.8 | 10.8 | 10.7 | 9.8 |
| 19 | 6.6 | 8.6 | 8.0 | 9.4 | 10.0 | 9.6 | 8.5 |
| 20 | 7.8 | 8.1 | 9.6 | 10.6 | 10.8 | 9.5 | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, R.; Nagano, N.; Hashimoto, S.; Saito, K.; Miyabayashi, H.; Noto, T.; Morioka, I. Three-Dimensional versus Two-Dimensional Evaluations of Cranial Asymmetry in Deformational Plagiocephaly Using a Three-Dimensional Scanner. Children 2022, 9, 788. https://doi.org/10.3390/children9060788
Kato R, Nagano N, Hashimoto S, Saito K, Miyabayashi H, Noto T, Morioka I. Three-Dimensional versus Two-Dimensional Evaluations of Cranial Asymmetry in Deformational Plagiocephaly Using a Three-Dimensional Scanner. Children. 2022; 9(6):788. https://doi.org/10.3390/children9060788
Chicago/Turabian StyleKato, Risa, Nobuhiko Nagano, Shin Hashimoto, Katsuya Saito, Hiroshi Miyabayashi, Takanori Noto, and Ichiro Morioka. 2022. "Three-Dimensional versus Two-Dimensional Evaluations of Cranial Asymmetry in Deformational Plagiocephaly Using a Three-Dimensional Scanner" Children 9, no. 6: 788. https://doi.org/10.3390/children9060788
APA StyleKato, R., Nagano, N., Hashimoto, S., Saito, K., Miyabayashi, H., Noto, T., & Morioka, I. (2022). Three-Dimensional versus Two-Dimensional Evaluations of Cranial Asymmetry in Deformational Plagiocephaly Using a Three-Dimensional Scanner. Children, 9(6), 788. https://doi.org/10.3390/children9060788






