Multiple Skeletal Anomalies of Sprague Dawley Rats following Prenatal Exposure to Anastatica hierochuntica, as Delineated by a Modified Double-Staining Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Anastatica hierochuntica Aqueous Extract
2.2. Animals
2.3. Chemicals
2.4. Experimental Design
2.4.1. Mating
2.4.2. Administration of Anastatica hierochuntica Aqueous Extract
2.4.3. Caesarian Section
2.4.4. Modified Double Skeletal Staining Procedure
Fixation of Full-Term Foetuses
Skinning and Evisceration
Dehydration
Alcian Blue Staining
Rehydration
Alizarin Red S Staining
Clearing I
Clearing II
Preservation
2.4.5. Skeletal Examination
Skull and Hyoid Bone Examination
Sternum Examination
Ribs Examination
Vertebral Column Examination
Forelimb and Hindlimb Examination
2.4.6. Statistical Analysis
3. Results
3.1. Skeletal Examination
3.1.1. Modified Staining Method
3.1.2. Thoracic Skeletal Examination
3.1.3. Skull Examination
3.1.4. Forelimbs Examination
3.1.5. Hindlimbs, Pelvic and Caudal Examination
3.2. Summary of Skeletal Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Law, K.S.; Soon, L.K.; Syed Mohsin, S.S.; Farid, C.G. Ultrastructural findings of Anastatica hierochuntica L. (Sanggul Fatimah) towards explaining its medicinal properties. Ann. Microsc. 2009, 9, 50–56. [Google Scholar]
- Daur, I. Chemical properties of the medicinal herb Kaff Maryam (Anastatica hierochuntica L.) and its relation to folk medicine use. Afr. J. Microbiol. Res. 2012, 23, 5048–5051. [Google Scholar]
- Kim, S.L.; Lean, K.S. Herbal medicines: Malaysian women’s knowledge and practice. Evid.-Based Complement. Altern. Med. 2013, 2013, 438139. [Google Scholar]
- Shah, A.H.; Bhandari, M.P.; Al-Harbi, N.O. Al-Ashban RM. Kaff-E-Maryam (Anastatica hierochuntica L.): Evaluation of gastro-protective activity and toxicity in different experimental models. Biol. Med. 2014, 6, 197–207. [Google Scholar]
- Mohamed, A.A.; Khalil, A.A.; El-Beltagi, H.E.S. Antioxidant and antimicrobial properties of kaff maryam (Anastatica hierochuntica) and doum palm (Hyphaene thebaica). Grasas Aceites 2010, 61, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Shaban, F.; Al-Azzawie, H.F.; Mohammed, A.S. Effect of alcoholic Anastatica hierochuntica extract on some biochemical and histological parameters in alloxan induced diabetic rats. Iraqi J. Sci. 2011, 52, 445–455. [Google Scholar]
- Zin, S.R.M.; Kassim, N.M.; Alshawsh, M.A.; Hashim, N.E.; Mohamed, Z. Biological activities of Anastatica hierochuntica L.: A systematic review. Biomed. Pharmacother. 2017, 91, 611–620. [Google Scholar] [CrossRef]
- Rasheed, R.A.; Bashir, A.K.; Ali, B.H. Fetal toxicity of Anastatica hierochuntica L. in mice. Fed. Am. Soc. Exp. Biol. 1997, 11, 2413. [Google Scholar]
- Fritz, H.; Hess, R. Ossification of the rat and mouse skeleton in the perinatal period. Teratology 1970, 4, 331–337. [Google Scholar] [CrossRef]
- Marques, N.F.Q.; Marques, A.P.B.M.; Iwano, A.L.; Golin, M.; De-Carvalho, R.R.; Paumgartten, F.J.R.; Dalsenter, P.R. Delayed ossification in Wistar rats induced by Morinda citrifolia L. exposure during pregnancy. J. Ethnopharmacol. 2010, 128, 85–91. [Google Scholar] [CrossRef]
- Sachetti, C.G.; de Carvalho, R.R.; Paumgartten, F.J.; Lameira, O.A.; Caldas, E.D. Developmental toxicity of copaiba tree (Copaifera reticulata Ducke, Fabaceae) oleoresin in rat. Food Chem. Toxicol. 2011, 49, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Rachmiel, A.; Even-Almos, M.; Aizenbud, D. Treatment of maxillary cleft palate: Distraction osteogenesis vs. Orthognathic surgery. Ann. Maxillofac. Surg. 2012, 2, 127–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oral Health Division, Ministry of Health of Malaysia. National Oral Health Survey of Schoolchildren 1997 (NOHSS ’97); Oral Health Division, Ministry of Health of Malaysia: Putrajaya, Malaysia, 1998. [Google Scholar]
- Ayu, A.; Samsudin, A.R.; Ismail, N.M.; Isa, M. Exposure to second-hand smoke and the risk of non-syndromic oral cleft among Malay children in Kelantan. Malays. J. Public Health Med. 2003, 3, 41–47. [Google Scholar]
- Rahman, R.A.; Ahmad, A.; Rahman, Z.A.; Mokhtar, K.I.; Lah, N.A.; Zilfalil, B.A.; Samsudin, A.R. Transforming growth factor-α and nonsyndromic cleft lip with or without palate or cleft palate only in Kelantan, Malaysia. Cleft Palate Craniofac. J. 2008, 45, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Alam, M.K.; Basri, R. Gene involvement in cleft lip and palate (CLP) patients. Bangl. J. Med. Sci. 2015, 14, 113–116. [Google Scholar] [CrossRef]
- Schultze, O. Uber Herstellung und conservirung durchsichtigen embryonen zum stadium der skeletbildung. Anat. Anz. 1897, 13, 3–5. [Google Scholar]
- Cumley, R.W.; Crow, J.F.; Griffen, A.B. Clearing specimens for the demonstration of bone. Stain Technol. 1939, 14, 7–11. [Google Scholar] [CrossRef]
- Dawson, A.B. A note on the staining of the skeleton of cleared specimens with Alizarin Red S. Stain Technol. 1926, 4, 123–124. [Google Scholar] [CrossRef]
- Gamble, J.T. A Combination bleaching-clearing agent and its use in the processing of “Spalteholz” preparations. Stain Technol. 1945, 20, 127–128. [Google Scholar] [CrossRef]
- Green, M.C. A rapid method for clearing and staining specimens for the demonstration of bone. Ohio. J. Sci. 1952, 52, 31–33. [Google Scholar]
- Lipman, H.J. Staining the skeleton of cleared embryos with Alizarin Red S. Stain Technol. 1935, 10, 61–63. [Google Scholar] [CrossRef]
- Mall, F.P. On ossification centers in human embryos less than one hundred days old. Dev. Dyn. 1906, 5, 433–458. [Google Scholar] [CrossRef] [Green Version]
- True, R.M. Staining of embryonic and small mammalian skeletal systems. Stain Technol. 1947, 22, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Van Wijhe, J.W. A new method for demonstrating cartilaginous microskeletons. K. Ned. Akad. van Wet. Proc. Ser. B Phys. Sci. 1902, 5, 47–51. [Google Scholar]
- Burdi, A.R. Toluidine blue-Alizarin Red S staining of cartilage and bone in whole-mount skeletons in vitro. Stain Technol. 1965, 40, 45–48. [Google Scholar] [CrossRef]
- Lundvall, H. Ueber demonstration embryonaler knorpelskelette. Anat. Anz. 1904, 25, 219–223. [Google Scholar]
- Rigueur, D.; Lyons, K.M. Whole-mount skeletal staining, Skeletal Development and Repair. Springer 2014, 13, 113–121. [Google Scholar]
- Ojeda, J.L.; Barbosa, E.; Bosque, P.G. Selective skeletal staining in whole chicken embryos; a rapid Alcian blue technique. Stain Technol. 1970, 45, 137–138. [Google Scholar]
- Inouye, M. Differential staining of cartilage and bone in fetal mouse skeleton by Alcian Blue and Alizarin Red S. Congenit. Anom. Res. Assoc. Japan 1976, 16, 171–173. [Google Scholar]
- Peker, T.; Kadioglu, D.; Erdogan, D. Visualisation of the fetal skeletal system by double staining with Alizarin Red and Alcian Blue. Gazi. Med. J. 1995, 6, 55–58. [Google Scholar]
- Igarashi, E.; Kawamura, N.; Okumura, H.; Hotta, K.; Okamoto, T.; Inaoka, M.; Takeshita, S.; Yasuda, M. Frequency of spontaneous axial skeletal variations detected by the double staining technique for ossified and cartilaginous skeleton in rat foetuses. Congenit. Anom. 1992, 32, 381–391. [Google Scholar] [CrossRef]
- Whitaker, J.; Dix, K.M. Double staining technique for rat foetus skeletons in teratological studies. Lab. Anim. 1979, 13, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Redfern, B.G.; Wise, L.D. High-throughput staining for the evaluation of fetal skeletal development in rats and rabbits. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2007, 80, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Depew, M.J. Analysis of skeletal ontogenesis through differential staining of bone and cartilage. Mol. Embryol. 2008, 461, 37–45. [Google Scholar]
- Ovchinnikov, D. Alcian Blue/Alizarin Red staining of cartilage and bone in mouse. Cold Spring Harb. Protoc. 2009, 3, 5170. [Google Scholar] [CrossRef]
- Fadel, R.; Sequeira, R.; Abu-Hijleh, M.; Obeidat, M.; Salem, A. Effect of prenatal administration of therapeutic doses of topiramate on ossification of ribs and vertebrae in rat foetuses. Rom. J. Morphol. Embryol. 2012, 53, 321–327. [Google Scholar]
- Sadeghi, F. Two separated protocols with the most important comments for skeletal staining in embryonic and adulthood period in laboratory animals. Anat. Sci. J. 2014, 11, 87–92. [Google Scholar]
- Salaramoli, J.; Sadeghi, F.; Gilanpour, H.; Azarnia, M.; Aliesfehani, T. Modified double skeletal staining protocols with Alizarin Red and Alcian Blue in laboratory animals. Ann. Mil. Health Sci. Res. 2015, 13, 76–81. [Google Scholar]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of Alizarin and Alizarin Red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef]
- Menegola, E.; Broccia, M.L.; Giavini, E. Atlas of rat fetal skeleton double stained for bone and cartilage. Teratology 2001, 64, 125–133. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.; Ryabov, M.A. Metal complexes with Alizarin and Alizarin Red S: Electronic absorption spectra and structure of ligands. Russ. J. Coord. Chem. 2004, 30, 365–370. [Google Scholar] [CrossRef]
- Meloan, S.N.; Puchtler, H.; Valentine, L.S. Alkaline and acid Alizarin Red S stains for alkali-soluble and alkali-insoluble calcium deposits. Arch. Pathol. 1972, 93, 190–197. [Google Scholar] [PubMed]
- Coleman, R. Development of the rat palate. Anat Rec 1965, 151, 107–117. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, V.; Nalbandian, J. Ultrastructure of mouse and rat palatal processes prior to and during secondary palate formation. Arch. Oral Biol. 1968, 13, 601–607. [Google Scholar] [CrossRef]
- Ferguson, M. Palatal shelf elevation in the Wistar rat fetus. J. Anat. 1978, 125, 555. [Google Scholar]
- Yoon, H.; Chung, I.S.; Seol, E.Y.; Park, B.Y.; Park, H.W. Development of the lip and palate in staged human embryos and early fetuses. Yonsei Med. J. 2000, 41, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Gritli-Linde, A. Molecular control of secondary palate development. Dev. Biol. 2007, 301, 309–326. [Google Scholar] [CrossRef] [Green Version]
- Gritli-Linde, A. The etiopathogenesis of cleft lip and cleft palate; usefulness and caveats of mouse models. Curr. Top. Dev. Biol. 2008, 84, 37–138. [Google Scholar]
- Enright, B.P.; Gu, Y.Z.; Snyder, R.D.; Dugyala, R.R.; Obert, L.A.; Treinen, K.A.; McIntyre, B.S.; Veneziale, R.W. Effects of the histamine H1 anatagonist chlorcyclizine on rat fetal palate development. Birth Defect. Res. Part B Dev. Reprod. Toxicol. 2010, 89, 474–484. [Google Scholar] [CrossRef]
- Danescu, A.; Mattson, M.; Dool, C.; Diewert, V.M.; Richman, J.M. Analysis of human soft palate morphogenesis supports regional regulation of palatal fusion. J. Anat. 2015, 227, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Deng, M.; Naluai-Cecchini, T.; Glass, I.A.; Cox, T.C. Differences in oral structure and tissue interactions during mouse vs. Human palatogenesis: Implications for the translation of findings from mice. Front. Physiol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicario, R.L. Evaluating Developmental Toxicity in the Rat as a Basis of Human Risk Assessment for Cleft Palate. Doctoral Dissertation, University of Manchester, Manchester, UK, 2018. [Google Scholar]
- Nanda, R.; Romeo, D. Differential cell proliferation of embryonic rat palatal processes as determined by incorporation of tritiated thymidine. Cleft Palate J. 1975, 12, 436–443. [Google Scholar] [PubMed]
- Omo-Aghoja, V.W.; Omo-Aghoja, L.; Ugboko, V.I.; Obuekwe, O.N.; Saheeb, B.D.O.; Feyi-Waboso, P.; Onowhakpor, A. Antenatal determinants of oro-facial clefts in Southern Nigeria. Afr. Health Sci. 2010, 10, 31–39. [Google Scholar] [PubMed]
- Loenarz, C.; Ge, W.; Coleman, M.L.; Rose, N.R.; Cooper, C.D.; Klose, R.J.; Ratcliffe, P.J.; Schofield, C.J. PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an N ε-dimethyl lysine demethylase. Hum. Mol. Genet. 2010, 19, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Gerenutti, M.; Oliveira, C.C.d.; Miranda, A.C.R.d.; Rosa, R.M.; Del Fiol, F.d.S. Reproductive performance and embriotoxicity of rats exposed to carbamazepine. Rev. Bras. De Ciências Farm. 2008, 44, 509–514. [Google Scholar] [CrossRef]
- Zin, S.R.M.; Kassim, N.M.; Mohamed, Z.; Fateh, A.H.; Alshawsh, M.A. Potential toxicity effects of Anastatica hierochuntica aqueous extract on prenatal development of Sprague-Dawley rats. J. Ethnopharmacol. 2019, 245, 112180. [Google Scholar] [CrossRef]
- Chernoff, N.; Rogers, J.M.; Turner, C.I.; Francis, B.M. Significance of supernumerary ribs in rodent developmental toxicity studies: Postnatal persistence in rats and mice. Toxicol. Sci. 1991, 17, 448–453. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose-Response 2014, 12, 13–20. [Google Scholar] [CrossRef]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Ninomiya, K.; Matsuda, H. Anastatins A and B, new skeletal flavonoids with hepatoprotective activities from the desert plant Anastatica hierochuntica. Bioorg. Med. Chem. Lett. 2003, 13, 1045–1049. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Härkönen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef]
- Van Meeuwen, J.A.; Korthagen, N.; De Jong, P.C.; Piersma, A.H.; Van den Berg, M. (Anti) estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol. Appl. Pharmacol. 2007, 221, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Van Meeuwen, J.A.; Nijmeijer, S.; Mutarapat, T.; Ruchirawat, S.; De Jong, P.C.; Piersma, A.H.; van den Berg, M. Aromatase inhibition by synthetic lactones and flavonoids in human placental microsomes and breast fibroblasts—A comparative study. Toxicol. Appl. Pharmacol. 2008, 228, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Modulation of aromatase by phytoestrogens. Enzym. Res. 2015, 2015, 594656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliaccio, S.I.L.V.I.A.; Newbold, R.R.; Bullock, B.C.; Jefferson, W.J.; Sutton, F.G., Jr.; McLachlan, J.A.; Korach, K.S. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology 1996, 137, 2118–2125. [Google Scholar] [CrossRef]
- Mahabady, M.K.; Gholami, M.R.; Varzi, H.N.; Zendedel, A.; Doostizadeh, M. Protective effect of quercetin on skeletal and neural tube teratogenicity induced by cyclophosphamide in rat foetuses. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2016; Volume 7, p. 133. [Google Scholar]
- Karampour, N.S.; Arzi, A.; Varzi, H.N.; Mohammadian, B.; Rezaei, M. Quercetin preventive effects on theophylline-induced anomalies in rat embryo. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e17834. [Google Scholar]
- Heidarinejad, S.; Khaksary, M.M.; Ranjbar, R.; Najafzadeh, V.H.; Mohammadian, B. Protective effect of quercetin on skeletal teratogenicity induced by experimental diabetes in rat foetus. Yafte 2017, 18, 48–58. [Google Scholar]
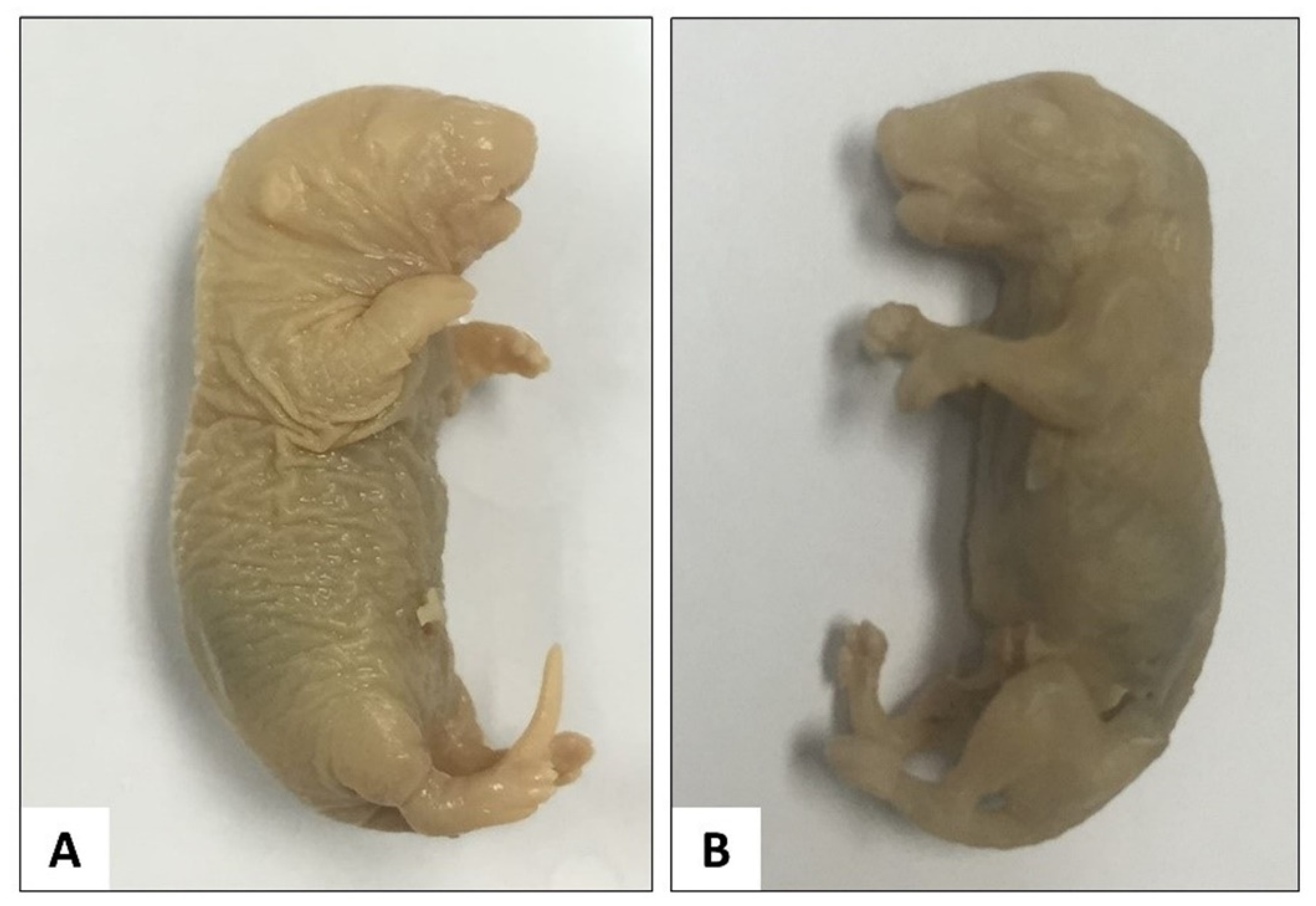

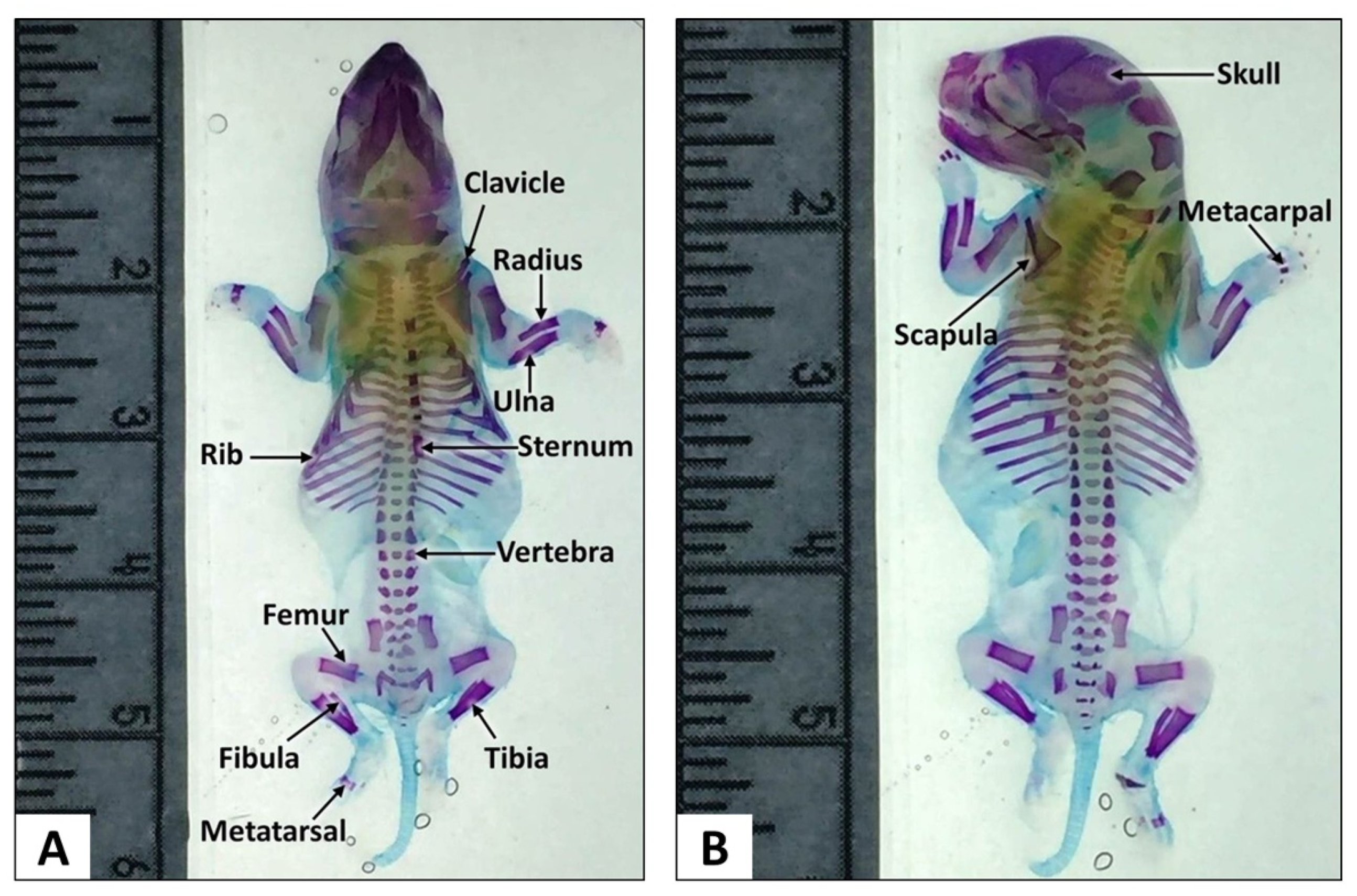
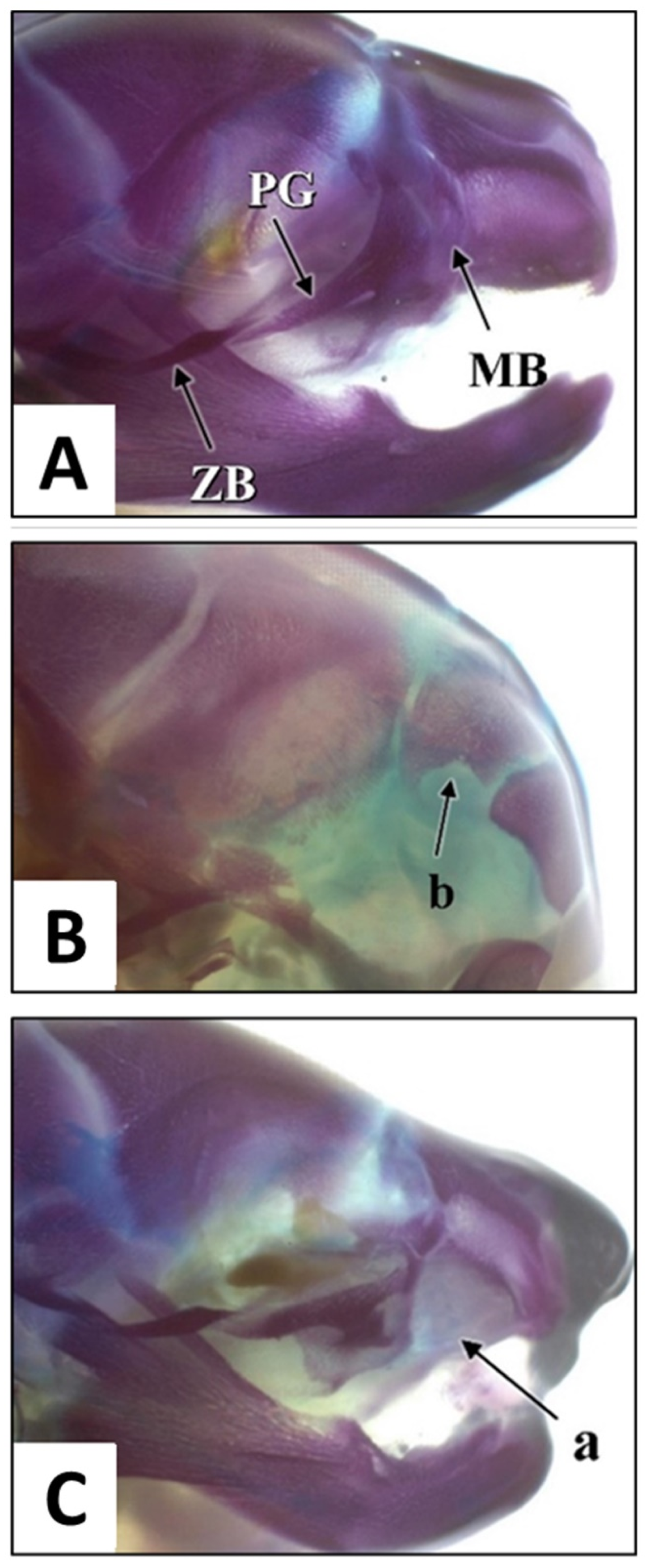

| No. | Step | Chemical | Duration |
|---|---|---|---|
| 1 | Fixation | 10% buffered formalin | 24 h |
| 70% ethanol | Until staining is commenced | ||
| 2 | Skinning | None | 5 to 15 min |
| 3 | Dehydration | 95% ethanol | ~24 h |
| Absolute (100%) ethanol | ~24 h | ||
| 4 | Alcian Blue staining | Alcian Blue 8GX (0.01%) | 12 to 24 h |
| 5 | Rehydration | 95% ethanol | 1 to 2 days |
| 70% ethanol | 1 to 2 days | ||
| Distilled water | 1 to 2 days | ||
| 6 | Alizarin Red S | Alizarin Red S (0.002%) | 12 to 24 h |
| 7 | Clearing I | 2% KOH | 12 to 24 h |
| 8 | Clearing II | 2% KOH and 85% glycerin | 3 to 5 days |
| 9 | Preservation | Glycerin and thymol | Storage |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| Control | 250 mg/kg AHAE | 500 mg/kg AHAE | 1000 mg/kg AHAE | ||
| Foetuses (litters) examined (N) | 30 (8) | 36 (8) | 40 (8) | 39 (8) | |
| Percentage of foetuses showing anomalies and (% pregnant female) in: | |||||
| Skull | |||||
| Os parietale | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Os frontale | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Os occipital | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Os interparietale | (IO) | 3.3 (12.5) | 22.2 (50) | 12.5 (50) | 2.56 (12.5) |
| Os supraoccipitale | (misshap.) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proc. Jugalis maxilla | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Os zygomatic | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Maxillary bone defect | 0 (0) | 0 (0) | 0 (0) | 2.56 (12.5) | |
| Hyoid bone | (absent/IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sternum | |||||
| All sternebrae | (split and misaligned) | 0 (0) | 0 (0) | 0 (0) | 2.56 (12.5) |
| (unossified) | 0 (0) | 0 (0) | 0 (0) | 7.7 (12.5) | |
| Sternebrae 1 | (split manubrium) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) |
| (IO) | 0 (0) | 2.78 (12.5) | 2.5 (12.5) | 0 (0) | |
| (absent) | 3.3 (12.5) | 0 (0) | 0 (0) | 0 (0) | |
| Sternebrae 2 | (misshap.) | 0 (0) | 0 (0) | 0 (0) | 2.56 (12.5) |
| (IO) | 0 (0) | 2.78 (12.5) | 2.5 (12.5) | 0 (0) | |
| (unossified) | 0 (0) | 2.78 (12.5) | 0 (0) | 2.56 (12.5) | |
| Sternebrae 3 | (misshap.) | 0 (0) | 2.78 (12.5) | 2.5 (12.5) | 10.3 (37.5) |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| (unossified) | 0 (0) | 2.78 (12.5) | 0 (0) | 2.56 (12.5) | |
| Sternebrae 4 | (misshap.) | 3.3 (12.5) | 2.78 (12.5) | 2.5 (12.5) | 10.3 (37.5) |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| (unossified) | 0 (0) | 2.78 (12.5) | 0 (0) | 2.56 (12.5) | |
| Sternebrae 5 | (misshap.) | 3.3 (12.5) | 0 (0) | 2.5 (12.5) | 2.56 (12.5) |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| (unossified) | 0 (0) | 2.78 (12.5) | 0 (0) | 5.1 (25) | |
| (absent) | 0 (0) | 8.3 (12.5) | 0 (0) | 0 (0) | |
| Sternebrae 6 | (split) | 0 (0) | 0 (0) | 0 (0) | 2.56 (12.5) |
| (unossified) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| (absent) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| Xiphisternum | (any abnormalities) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Foetuses (litters) examined (N) | 30 (8) | 36 (8) | 40 (8) | 39 (8) | |
| Ribs and vertebrae | |||||
| All ribs | (misshap.) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) |
| (Fused) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| (Wavy: 1 pair) | 3.3 (12.5) | 0 (0) | 0 (0) | 0 (0) | |
| 13th rib | (short) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 14th rib/ rudimentary | (both sides) | 13.3 (37.5) | 5.6 (25) | 10 (25) | 2.56 (12.5) |
| (one side) | 10 (25) | 8.3 (37.5) | 2.5 (12.5) | 0 (0) | |
| 3rd lumbar rib | (both sides) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) |
| 4th lumbar rib | (both sides) | 6.7 (25) | 5.6 (25) | 0 (0) | 0 (0) |
| (one side) | 3.3 (12.5) | 0 (0) | 0 (0) | 0 (0) | |
| Atlas | (misshap.) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Thoracic vert. OC | (dumbbell) | 16.7 (37.5) | 36.1 (75) | 25 (75) | 41 (75) |
| (bipartite) | 10 (25) | 13.9 (50) | 17.5 (62.5) | 15.4 (37.5) | |
| (IO) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| Lumbar vert. OC | (dumbbell) | 3.3 (12.5) | 0 (0) | 2.5 (12.5) | 0 (0) |
| (bipartite) | 0 (0) | 0 (0) | 5 (25) | 0 (0) | |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 5.1 (12.5) | |
| (absent) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| Sacral | (IO) | 0 (0) | 0 (0) | 0 (0) | 10.3 (12.5) |
| (absent) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| Caudal | (absent) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) |
| Limbs and pelvic bones | |||||
| All forelimbs | (short) | 0 (0) | 0 (0) | 0 (0) | 10.3 (12.5) |
| Metacarpals | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Os humerus | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ilium | (shortened) | 0 (0) | 0 (0) | 0 (0) | 10.3 (12.5) |
| Pubis and ischium | (shortened) | 0 (0) | 0 (0) | 0 (0) | 5.1 (12.5) |
| (unossified) | 0 (0) | 0 (0) | 0 (0) | 5.1 (12.5) | |
| (misshap.) | 0 (0) | 2.78 (12.5) | 0 (0) | 0 (0) | |
| All hindlimbs | (short) | 0 (0) | 0 (0) | 0 (0) | 10.3 (12.5) |
| Os femur | (misshap.) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Tibia and fibula | (misshap.) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Metatarsals | (IO) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin, S.R.M.; Alshawsh, M.A.; Mohamed, Z. Multiple Skeletal Anomalies of Sprague Dawley Rats following Prenatal Exposure to Anastatica hierochuntica, as Delineated by a Modified Double-Staining Method. Children 2022, 9, 763. https://doi.org/10.3390/children9050763
Zin SRM, Alshawsh MA, Mohamed Z. Multiple Skeletal Anomalies of Sprague Dawley Rats following Prenatal Exposure to Anastatica hierochuntica, as Delineated by a Modified Double-Staining Method. Children. 2022; 9(5):763. https://doi.org/10.3390/children9050763
Chicago/Turabian StyleZin, Siti Rosmani Md, Mohammed Abdullah Alshawsh, and Zahurin Mohamed. 2022. "Multiple Skeletal Anomalies of Sprague Dawley Rats following Prenatal Exposure to Anastatica hierochuntica, as Delineated by a Modified Double-Staining Method" Children 9, no. 5: 763. https://doi.org/10.3390/children9050763
APA StyleZin, S. R. M., Alshawsh, M. A., & Mohamed, Z. (2022). Multiple Skeletal Anomalies of Sprague Dawley Rats following Prenatal Exposure to Anastatica hierochuntica, as Delineated by a Modified Double-Staining Method. Children, 9(5), 763. https://doi.org/10.3390/children9050763








