Evaluating Providers’ Prescription Opioid Instructions to Pediatric Patients
Abstract
1. Introduction
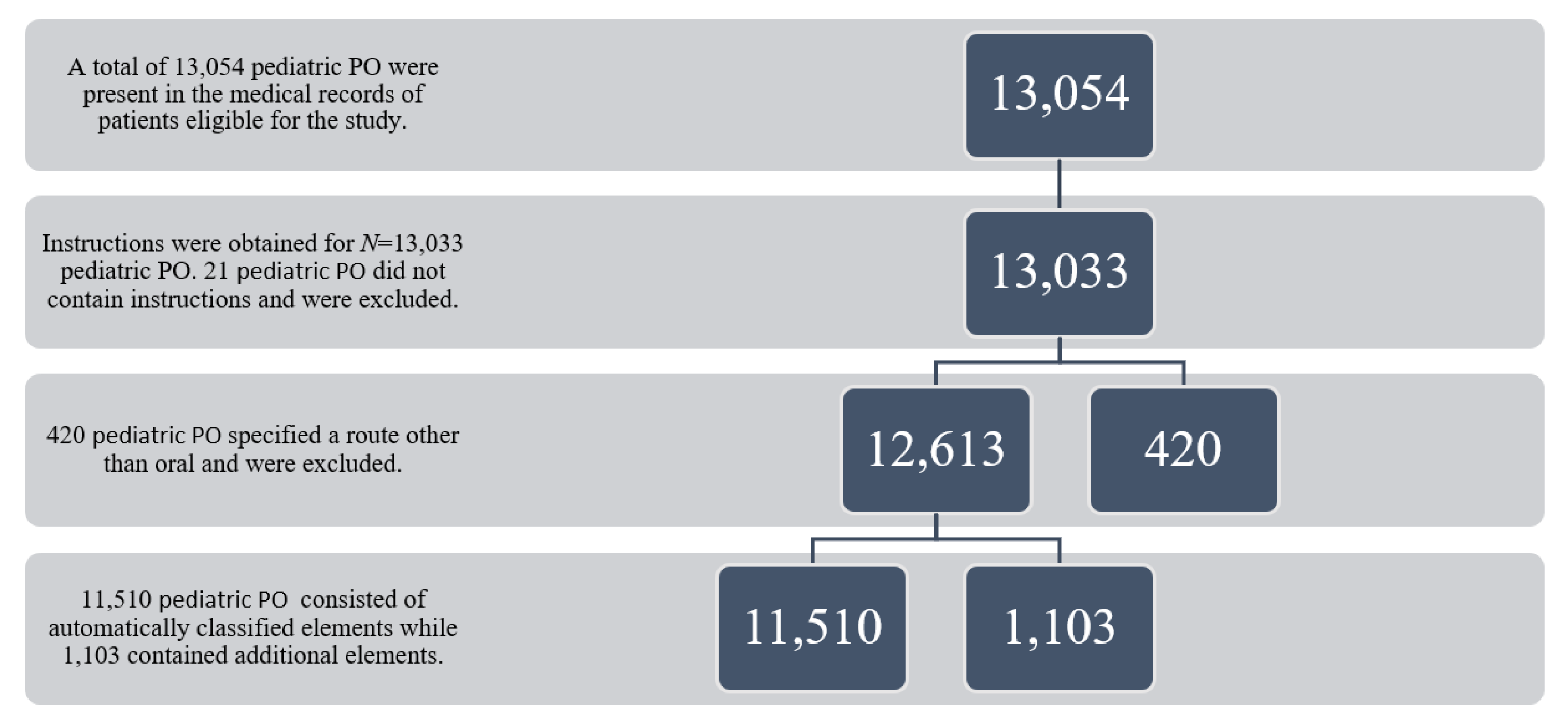
2. Methods
2.1. Procedures
2.2. Classification of Text Features and Data Processing
2.3. Statistical Analysis
3. Results
3.1. Pediatric PO Patient Characteristics
3.2. Pediatric PO Instructions
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miech, R.; Johnston, L.; O’Malley, P.M.; Keyes, K.M.; Heard, K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics 2015, 136, e1169–e1177. [Google Scholar] [CrossRef] [PubMed]
- McCabe, S.E.; West, B.T.; Morales, M.; Cranford, J.A.; Boyd, C.J. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction 2007, 102, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.T.; McCauley, J.L.; Back, S.E. Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update. Am. J. Psychiatry 2016, 173, 18–26. [Google Scholar] [CrossRef] [PubMed]
- McCabe, S.E.; West, B.T.; Veliz, P.; McCabe, V.V.; Stoddard, S.A.; Boyd, C.J. Trends in Medical and Nonmedical Use of Prescription Opioids Among US Adolescents: 1976–2015. Pediatrics 2017, 139, e20162387. [Google Scholar] [CrossRef]
- Ferland, K.E.; Vega, E.; Ingelmo, P.M. Acute pain management in children: Challenges and recent improvements. Curr. Opin. Anesthesiol. 2018, 31, 327–332. [Google Scholar] [CrossRef]
- Jones, C.M.; Clayton, H.B.; Deputy, N.P.; Roehler, D.R.; Ko, J.Y.; Esser, M.B.; Brookmeyer, K.A.; Hertz, M.F. Prescription Opioid Misuse and Use of Alcohol and Other Substances Among High School Students—Youth Risk Behavior Survey, United States, 2019. MMWR Suppl. 2020, 69, 38–46. [Google Scholar] [CrossRef]
- Wilkins, N.J.; Clayton, H.; Jones, C.M.; Brown, M. Current Prescription Opioid Misuse and Suicide Risk Behaviors Among High School Students. Pediatrics 2021, 147, e2020030601. [Google Scholar] [CrossRef]
- Baiden, P.; Graaf, G.; Zaami, M.; Acolatse, C.K.; Adeku, Y. Examining the association between prescription opioid misuse and suicidal behaviors among adolescent high school students in the United States. J. Psychiatr. Res. 2019, 112, 44–51. [Google Scholar] [CrossRef]
- As-Sanie, S.; Till, S.R.; Mowers, E.L.; Lim, C.S.; Skinner, B.D.; Fritsch, L.; Tsodikov, A.; Dalton, V.K.; Clauw, D.J.; Brummett, C.M. Opioid Prescribing Patterns, Patient Use, and Postoperative Pain After Hysterectomy for Benign Indications. Obstet. Gynecol. 2017, 130, 1261–1268. [Google Scholar] [CrossRef]
- Hill, M.V.; McMahon, M.L.; Stucke, R.S.; Barth, R.J. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann. Surg. 2017, 265, 709–714. [Google Scholar] [CrossRef]
- Bicket, M.C.; Long, J.J.; Pronovost, P.J.; Alexander, G.C.; Wu, C.L. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg. 2017, 152, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Monitto, C.L.; Hsu, A.; Gao, S.; Vozzo, P.T.; Park, P.S.; Roter, D.; Yenokyan, G.; White, E.D.; Kattail, D.; Edgeworth, A.E.; et al. Opioid Prescribing for the Treatment of Acute Pain in Children on Hospital Discharge. Anesth. Analg. 2017, 125, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.C.; Wolf, M.S.; Lopez, A.; Russell, A.; Chen, A.H.; Schillinger, D.; Moy, G.; Sarkar, U. Expanding the Universal Medication Schedule: A patient-centred approach. BMJ Open 2014, 4, e003699. [Google Scholar] [CrossRef] [PubMed]
- Grissinger, M. “Use as Directed” Can Cause Confusion for Both Patients and Practitioners. Pharm. Ther. 2019, 44, 168–169. [Google Scholar]
- Wolf, M.S.; Davis, T.C.; Curtis, L.M.; Webb, J.A.; Bailey, S.C.; Shrank, W.H.; Lindquist, L.; Ruo, B.; Bocchini, M.V.; Parker, R.M.; et al. Effect of standardized, patient-centered label instructions to improve comprehension of prescription drug use. Med. Care 2011, 49, 96–100. [Google Scholar] [CrossRef]
- Bailey, S.C.; Sarkar, U.; Chen, A.H.; Schillinger, D.; Wolf, M.S. Evaluation of language concordant, patient-centered drug label instructions. J. Gen. Intern. Med. 2012, 27, 1707–1713. [Google Scholar] [CrossRef]
- Koster, E.S.; Blom, L.; Winters, N.A.; Van Hulten, R.P.; Bouvy, M.L. Interpretation of drug label instructions: A study among four immigrants groups in the Netherlands. Int. J. Clin. Pharm. 2014, 36, 274–281. [Google Scholar] [CrossRef]
- Bailey, S.C.; Navaratnam, P.; Black, H.; Russell, A.L.; Wolf, M.S. Advancing Best Practices for Prescription Drug Labeling. Ann. Pharm. 2015, 49, 1222–1236. [Google Scholar] [CrossRef]
- Kripalani, S. Structured prescription instructions and medication adherence. Am. J. Health Syst. Pharm. 2020, 77, 157–158. [Google Scholar] [CrossRef]
- McCarthy, D.M.; Davis, T.C.; King, J.P.; Mullen, R.J.; Bailey, S.C.; Serper, M.; Jacobson, K.L.; Parker, R.M.; Wolf, S.M. Take-Wait-Stop: A patient-centered strategy for writing PRN medication instructions. J. Health Commun. 2013, 18 (Suppl. S1), 40–48. [Google Scholar] [CrossRef][Green Version]
- Wolf, M.S.; Davis, T.C.; Curtis, L.M.; Bailey, S.C.; Knox, J.P.; Bergeron, A.; Abbet, M.; Shrank, W.H.; Parker, R.M.; Wood, A.J.J. A Patient-Centered Prescription Drug Label to Promote Appropriate Medication Use and Adherence. J. Gen. Intern. Med. 2016, 31, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Karmiy, S.J.; Forsythe, K.J.; Amato, M.G.; Wright, A.; Lai, K.H.; Lambert, B.L.; Liebovitz, D.M.; Eguale, T.; Volk, L.A.; et al. How often do prescribers include indications in drug orders? Analysis of 4 million outpatient prescriptions. Am. J. Health Syst. Pharm 2019, 76, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.; Mirica, M.M.; Dhavle, A.A.; Galanter, W.L.; Lambert, B.; Wright, A. A Prescription For Enhancing Electronic Prescribing Safety. Health Aff. (Millwood) 2018, 37, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Federman, A.; Sarzynski, E.; Brach, C.; Francaviglia, P.; Jacques, J.; Jandorf, L.; Munoz, A.S.; Wolf, M.; Kannry, J. Challenges optimizing the after visit summary. Int. J. Med. Inform. 2018, 120, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kron, K.; Myers, S.; Volk, L.; Nathan, A.; Neri, P.; Salazar, A.; Amato, M.G.; Wright, A.; Karmiy, S.; McCord, S.; et al. Incorporating medication indications into the prescribing process. Am. J. Health Syst. Pharm. 2018, 75, 774–783. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, Y.; Dorsch, M.P.; Ding, Y.; Vydiswaran, V.V.; Lester, C.A. Work effort, readability and quality of pharmacy transcription of patient directions from electronic prescriptions: A retrospective observational cohort analysis. BMJ Qual. Saf. 2020, 30, 311–319. [Google Scholar] [CrossRef]
- Silvers, J.A.; Squeglia, L.M.; Rømer Thomsen, K.; Hudson, K.A.; Feldstein Ewing, S.W. Hunting for What Works: Adolescents in Addiction Treatment. Alcohol. Clin. Exp. Res. 2019, 43, 578–592. [Google Scholar] [CrossRef]
- U.S. Department of Education. The NAEP Reading Achievement Levels by Grade; US Department of Education: Washington, DC, USA, 2011.
- Cotugna, N.; Vickery, C.E.; Carpenter-Haefele, K.M. Evaluation of literacy level of patient education pages in health-related journals. J. Community Health 2005, 30, 213–219. [Google Scholar] [CrossRef]
- MedlinePlus. How to Write Easy-to-Read Health Materials; U.S. National Library of Medicine, National Institutes of Health: Bethesda, MD, USA, 2013.
- Locke, M.R.; Shiyanbola, O.O.; Gripentrog, E. Improving prescription auxiliary labels to increase patient understanding. J. Am. Pharm. Assoc. 2014, 54, 267–274. [Google Scholar] [CrossRef]
- Hudgins, J.D.; Porter, J.J.; Monuteaux, M.C.; Bourgeois, F.T. Prescription opioid use and misuse among adolescents and young adults in the United States: A national survey study. PLoS Med. 2019, 16, e1002922. [Google Scholar] [CrossRef]
- Hall, W.J.; Chapman, M.V.; Lee, K.M.; Merino, Y.M.; Thomas, T.W.; Payne, B.K.; Eng, E.; Day, S.H.; Coyne-Beasley, T. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. Am. J. Public Health 2015, 105, e60–e76. [Google Scholar] [CrossRef] [PubMed]
| N (%) | M (SD) | |
|---|---|---|
| Age | 11.1 (5.5) | |
| Gender | ||
| Female | 4650 (41.5%) | |
| Male | 6563 (58.5%) | |
| Race | ||
| non-Hispanic White | 8747 (78.0%) | |
| African American | 255 (2.3%) | |
| Asian | 355 (3.2%) | |
| Multiracial | 916 (8.2%) | |
| Unknown or not reported | 940 (8.4%) | |
| Ethnicity | ||
| Hispanic | 2032 (18.1%) | |
| Not Hispanic | 8625 (79.6%) | |
| Unknown or not reported | 556 (5.0%) | |
| Primary language | ||
| English | 10,219 (91.1%) | |
| Spanish | 733 (6.5%) | |
| Other | 169 (1.6%) | |
| Unknown | 92 (0.8%) |
| N (%) with element | |
|---|---|
| Amount of pediatric PO to take | 12,538 (99.4%) |
| Discrete amount | 10,664 (85.1%) |
| Range | 2474 (14.9%) |
| Amount as numeral | 12,508 (99.8%) |
| Amount as word | 30 (0.2%) |
| Frequency of pediatric PO to take | 12,542 (99.4%) |
| Specified frequency (every # hours) | 12,365 (98.6%) |
| Range (every # hours) | 107 (0.9%) |
| Specified frequency (# per day) | 69 (0.6%) |
| Range (# per day) | 1 (0.0%) |
| Route of pediatric PO administration | 12,534 (99.4%) |
| “As needed” | 12,369 (98.1%) |
| Pediatric PO indication specified | 9954 (78.9%) |
| Severity of pain specified | 9171 (92.1%) |
| Cause or location of pain specified | 235 (2.4%) |
| Additional pediatric PO instructions | 1534 (12.2%) |
| Instructions to limit pediatric PO medication | 1161 (9.2%) |
| Maximum dosing frequency | 12 (1.0%) |
| Maximum total amount | 477 (38.5%) |
| Maximum duration for pediatric PO use | 400 (34.5%) |
| Direction to use non-PO medication first | 304 (26.2%) |
| Instruction to minimize amount of pediatric PO | 19 (1.6%) |
| Instructions to wean pediatric PO | 142 (1.1%) |
| Weaning steps specified for pediatric PO | 68 (47.9%) |
| Potentially confusing phrasing | 2443 (19.4%) |
| Prescribing Department | Patient Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Surgical | Non-Surgical | OR | 95% CI | ≥13 Years | <13 Years | OR | 95% CI |
| N (%) with specified discrete amount of pediatric PO | 2714 (76.6%) | 7349 (81.0%) | 0.77 *** | [0.70, 0.85] | 3027 (70.3%) | 7037 (84.7%) | 2.35 *** | [2.15, 2.57] |
| N (%) with instructions to limit pediatric PO | 454 (12.8%) | 707 (7.8%) | 1.74 *** | [1.53, 1.97] | 354 (15.3%) | 807 (9.7%) | 1.20 *** | [1.05, 1.37] |
| N (%) with severity of pain specified | 3016 (85.1%) | 6154 (67.9%) | 2.72 *** | [2.45, 3.02] | 3122 (72.4%) | 6049 (72.8%) | 1.02 | [0.94, 1.11] |
| N (%) with potentially confusing phrasing on pediatric PO | 587 (16.6%) | 1856 (20.5%) | 0.77 *** | [0.70, 0.86] | 945 (21.9%) | 1498 (18.0%) | 0.78 *** | [0.71, 0.86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, D.D.; Brown, P.C.M.; Murphy, C.; Ho, D.; Hudson, K.A.; Wilson, A.C.; Feldstein Ewing, S.W. Evaluating Providers’ Prescription Opioid Instructions to Pediatric Patients. Children 2022, 9, 707. https://doi.org/10.3390/children9050707
Tran DD, Brown PCM, Murphy C, Ho D, Hudson KA, Wilson AC, Feldstein Ewing SW. Evaluating Providers’ Prescription Opioid Instructions to Pediatric Patients. Children. 2022; 9(5):707. https://doi.org/10.3390/children9050707
Chicago/Turabian StyleTran, Denise D., Patrick C. M. Brown, Corrin Murphy, Diana Ho, Karen A. Hudson, Anna C. Wilson, and Sarah W. Feldstein Ewing. 2022. "Evaluating Providers’ Prescription Opioid Instructions to Pediatric Patients" Children 9, no. 5: 707. https://doi.org/10.3390/children9050707
APA StyleTran, D. D., Brown, P. C. M., Murphy, C., Ho, D., Hudson, K. A., Wilson, A. C., & Feldstein Ewing, S. W. (2022). Evaluating Providers’ Prescription Opioid Instructions to Pediatric Patients. Children, 9(5), 707. https://doi.org/10.3390/children9050707






