COVID-19 Vaccine—A Potential Trigger for MOGAD Transverse Myelitis in a Teenager—A Case Report and a Review of the Literature
Abstract
:1. Introduction
2. Case Report
2.1. Presenting Concerns
2.2. Clinical Findings
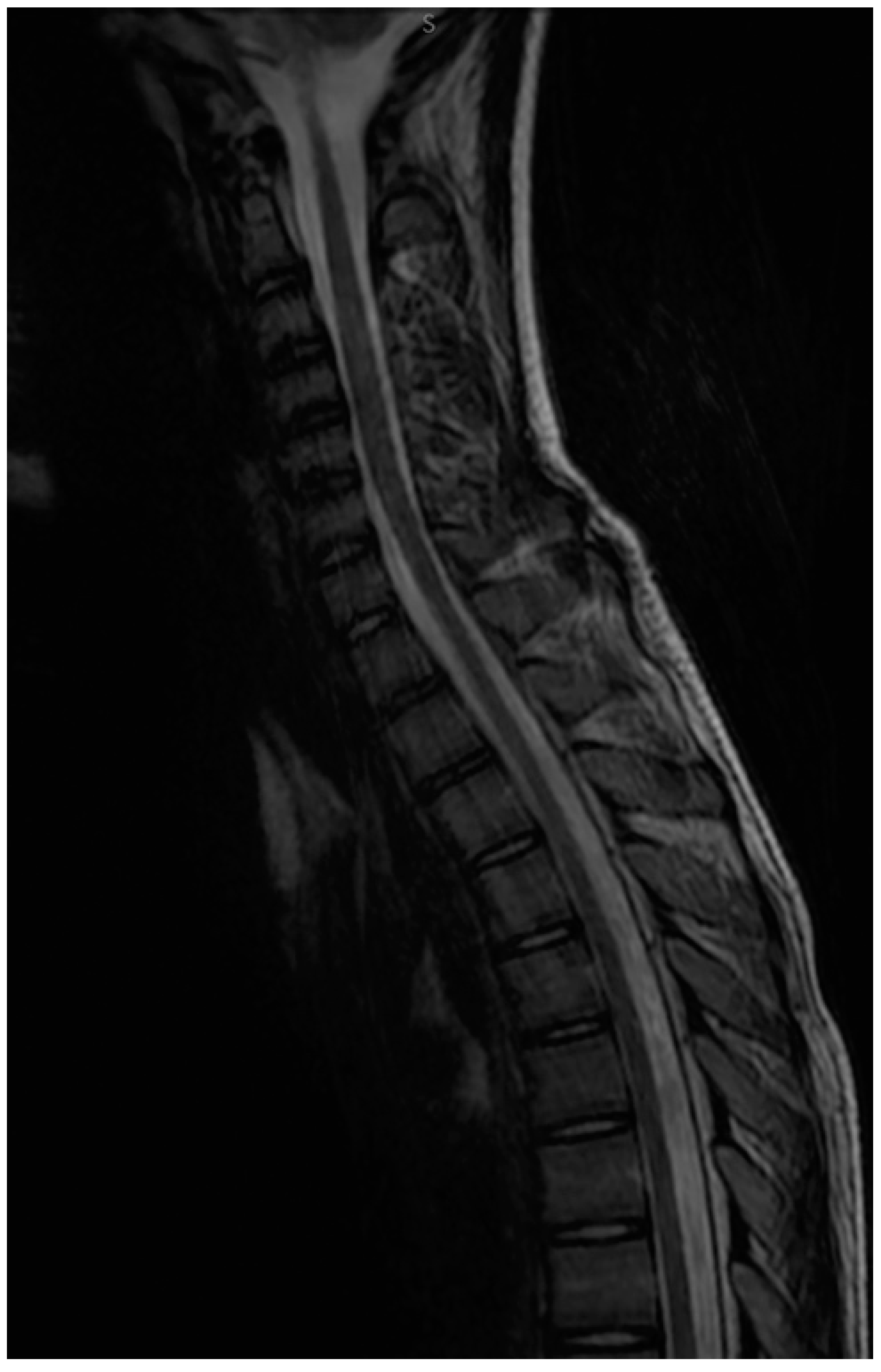
2.3. Diagnostic Focus and Assessment
2.4. Therapeutic Focus and Assessment
2.5. Follow-up and Monitoring
3. Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrosius, W.; Michalak, S.; Kozubski, W.; Kalinowska, A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management. Int. J. Mol. Sci. 2020, 22, 100. [Google Scholar] [CrossRef] [PubMed]
- Hilton, A.A.; Slavin, A.J.; Hilton, D.J.; Bernard, C.C. Characterization of cDNA and genomic clones encoding human myelin oligodendrocyte glycoprotein. J. Neurochem. 1995, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Di Pauli, F.; Rostásy, K.; Berger, T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat. Rev. Neurol. 2013, 9, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hennes, E.-M.; Baumann, M.; Lechner, C.; Rostásy, K. MOG Spectrum Disorders and Role of MOG-Antibodies in Clinical Practice. Neuropediatrics 2018, 49, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ketelslegers, I.A.; Van Pelt, D.E.; Bryde, S.; Neuteboom, R.F.; Catsman-Berrevoets, C.E.; Hamann, D.; Hintzen, R.Q. Anti-MOG antibodies plead against MS diagnosis in an Acquired Demyelinating Syndromes cohort. Mult. Scler. Houndmills Basingstoke Engl. 2015, 21, 1513–1520. [Google Scholar] [CrossRef]
- Hacohen, Y.; Absoud, M.; Deiva, K.; Hemingway, C.; Nytrova, P.; Woodhall, M.; Palace, J.; Wassmer, E.; Tardieu, M.; Vincent, A.; et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e81. [Google Scholar] [CrossRef] [Green Version]
- Hennes, E.-M.; Baumann, M.; Schanda, K.; Anlar, B.; Bajer-Kornek, B.; Blaschek, A.; Brantner-Inthaler, S.; Diepold, K.; Eisenkölbl, A.; Gotwald, T.; et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 2017, 89, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Waters, P.; Fadda, G.; Woodhall, M.; O’Mahony, J.; Brown, R.A.; Castro, D.A.; Longoni, G.; Irani, S.R.; Sun, B.; Yeh, E.A.; et al. Serial Anti-Myelin Oligodendrocyte Glycoprotein Antibody Analyses and Outcomes in Children With Demyelinating Syndromes. JAMA Neurol. 2020, 77, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, S.; Dale, R.C.; Brilot, F. Anti-MOG antibody: The history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun. Rev. 2016, 15, 307–324. [Google Scholar] [CrossRef]
- De Mol, C.L.; Wong, Y.; van Pelt, E.D.; Wokke, B.; Siepman, T.; Neuteboom, R.F.; Hamann, D.; Hintzen, R.Q. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult. Scler. Houndmills Basingstoke Engl. 2020, 26, 806–814. [Google Scholar] [CrossRef]
- Reindl, M.; Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019, 15, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Bruijstens, A.L.; Lechner, C.; Flet-Berliac, L.; Deiva, K.; Neuteboom, R.F.; Hemingway, C.; Wassmer, E.; E.U. paediatric MOG consortium; Baumann, M.; Bartels, F.; et al. E.U. paediatric MOG consortium consensus: Part 1—Classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2020, 29, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Jurynczyk, M.; Messina, S.; Woodhall, M.R.; Raza, N.; Everett, R.; Roca-Fernandez, A.; Tackley, G.; Hamid, S.; Sheard, A.; Reynolds, G.; et al. Clinical presentation and prognosis in MOG-antibody disease: A UK study. Brain J. Neurol. 2017, 140, 3128–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senanayake, B.; Jitprapaikulsan, J.; Aravinthan, M.; Wijesekera, J.C.; Ranawaka, U.K.; Riffsy, M.T.; Paramanathan, T.; Sagen, J.; Fryer, J.P.; Schmeling, J.; et al. Seroprevalence and clinical phenotype of MOG-IgG-associated disorders in Sri Lanka. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1381–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, D.; Pittock, S.J.; Krecke, K.N.; Morris, P.P.; Sechi, E.; Zalewski, N.L.; Weinshenker, B.G.; Shosha, E.; Lucchinetti, C.F.; Fryer, J.P.; et al. Clinical, Radiologic, and Prognostic Features of Myelitis Associated with Myelin Oligodendrocyte Glycoprotein Autoantibody. JAMA Neurol. 2019, 76, 301–309. [Google Scholar] [CrossRef]
- Bartels, F.; Lu, A.; Oertel, F.C.; Finke, C.; Paul, F.; Chien, C. Clinical and neuroimaging findings in MOGAD-MRI and OCT. Clin. Exp. Immunol. 2021, 206, 266–281. [Google Scholar] [CrossRef]
- Solomon, J.M.; Paul, F.; Chien, C.; Oh, J.; Rotstein, D.L. A window into the future? MRI for evaluation of neuromyelitis optica spectrum disorder throughout the disease course. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211014388. [Google Scholar] [CrossRef]
- Sechi, E.; Krecke, K.N.; Pittock, S.J.; Dubey, D.; Lopez-Chiriboga, A.S.; Kunchok, A.; Weinshenker, B.G.; Zalewski, N.L.; Flanagan, E.P. Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult. Scler. Houndmills Basingstoke Engl. 2021, 27, 303–308. [Google Scholar] [CrossRef]
- Jarius, S.; Kleiter, I.; Ruprecht, K.; Asgari, N.; Pitarokoili, K.; Borisow, N.; Hümmert, M.W.; Trebst, C.; Pache, F.; Winkelmann, A.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 3: Brainstem involvement—Frequency, presentation and outcome. J. Neuroinflamm. 2016, 13, 281. [Google Scholar] [CrossRef] [Green Version]
- Jarius, S.; Paul, F.; Aktas, O.; Asgari, N.; Dale, R.C.; de Seze, J.; Franciotta, D.; Fujihara, K.; Jacob, A.; Kim, H.J.; et al. MOG encephalomyelitis: International recommendations on diagnosis and antibody testing. J. Neuroinflamm. 2018, 15, 134. [Google Scholar] [CrossRef]
- Hor, J.Y.; Asgari, N.; Nakashima, I.; Broadley, S.A.; Leite, M.I.; Kissani, N.; Jacob, A.; Marignier, R.; Weinshenker, B.G.; Paul, F.; et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Front. Neurol. 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Calvo, A.; Ruiz, A.; Maillart, E.; Audoin, B.; Zephir, H.; Bourre, B.; Ciron, J.; Collongues, N.; Brassat, D.; Cotton, F.; et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology 2018, 90, e1858–e1869. [Google Scholar] [CrossRef] [PubMed]
- Netravathi, M.; Holla, V.V.; Nalini, A.; Yadav, R.; Vengalil, S.; Oommen, A.T.; Reshma, S.S.; Kamble, N.; Thomas, P.T.; Maya, B.; et al. Myelin oligodendrocyte glycoprotein-antibody-associated disorder: A new inflammatory CNS demyelinating disorder. J. Neurol. 2021, 268, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.-A.; Hümmert, M.W.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 2016, 13, 280. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Alhasan, S.; Vogels, C.B.F.; Grubaugh, N.D.; Farhadian, S.; Longbrake, E.E. MOG-associated encephalitis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2021, 50, 102857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jones-Lopez, E.C.; Soneji, D.J.; Azevedo, C.J.; Patel, V.R. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Optic Neuritis and Myelitis in COVID-19. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2020, 40, 398–402. [Google Scholar] [CrossRef]
- De Ruijter, N.S.; Kramer, G.; Gons, R.A.R.; Hengstman, G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: A case report. Mult. Scler. Relat. Disord. 2020, 46, 102474. [Google Scholar] [CrossRef]
- Escolà, J.K.; Deuschl, C.; Junker, A.; Dusse, F.; Pul, R.; Kleinschnitz, C.; Köhrmann, M.; Frank, B. MOG antibody-associated encephalomyelitis mimicking bacterial meningomyelitis following ChAdOx1 nCoV-19 vaccination: A case report. Ther. Adv. Neurol. Disord. 2022, 15, 17562864211070684. [Google Scholar] [CrossRef]
- Chen, S.; Fan, X.-R.; He, S.; Zhang, J.-W.; Li, S.-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 3537–3539. [Google Scholar] [CrossRef]
- Helmchen, C.; Buttler, G.M.; Markewitz, R.; Hummel, K.; Wiendl, H.; Boppel, T. Acute bilateral optic/chiasm neuritis with longitudinal extensive transverse myelitis in longstanding stable multiple sclerosis following vector-based vaccination against the SARS-CoV-2. J. Neurol. 2022, 269, 49–54. [Google Scholar] [CrossRef]
- Hintzen, R.Q.; Dale, R.C.; Neuteboom, R.F.; Mar, S.; Banwell, B. Pediatric acquired CNS demyelinating syndromes: Features associated with multiple sclerosis. Neurology 2016, 87, S67–S73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, S.; Pardo, S.; Levy, M. Clinical characteristics of myelin oligodendrocyte glycoprotein antibody neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2019, 30, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lechner, C.; Baumann, M.; Hennes, E.-M.; Schanda, K.; Marquard, K.; Karenfort, M.; Leiz, S.; Pohl, D.; Venkateswaran, S.; Pritsch, M.; et al. Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.K.; Callegaro, D.; Lana-Peixoto, M.A.; Waters, P.J.; de Haidar Jorge, F.M.; Takahashi, T.; Nakashima, I.; Apostolos-Pereira, S.L.; Talim, N.; Simm, R.F.; et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014, 82, 474–481. [Google Scholar] [CrossRef]
- Tantsis, E.M.; Prelog, K.; Alper, G.; Benson, L.; Gorman, M.; Lim, M.; Mohammad, S.S.; Ramanathan, S.; Brilot, F.; Dale, R.C.; et al. Magnetic resonance imaging in enterovirus-71, myelin oligodendrocyte glycoprotein antibody, aquaporin-4 antibody, and multiple sclerosis-associated myelitis in children. Dev. Med. Child Neurol. 2019, 61, 1108–1116. [Google Scholar] [CrossRef]
- Ogawa, R.; Nakashima, I.; Takahashi, T.; Kaneko, K.; Akaishi, T.; Takai, Y.; Sato, D.K.; Nishiyama, S.; Misu, T.; Kuroda, H.; et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e322. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; ZhangBao, J.; Zhou, L.; Zhang, Y.; Li, H.; Li, Y.; Huang, Y.; Wang, M.; Lu, C.; Lu, J.; et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: A single-center cohort study in Shanghai, China. Eur. J. Neurol. 2019, 26, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Cobo-Calvo, A.; Sepúlveda, M.; Rollot, F.; Armangué, T.; Ruiz, A.; Maillart, E.; Papeix, C.; Audoin, B.; Zephir, H.; Biotti, D.; et al. Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J. Neuroinflamm. 2019, 16, 134. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, S.; Mohammad, S.; Tantsis, E.; Nguyen, T.K.; Merheb, V.; Fung, V.S.C.; White, O.B.; Broadley, S.; Lechner-Scott, J.; Vucic, S.; et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J. Neurol. Neurosurg. Psychiatry 2018, 89, 127–137. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărginean, C.O.; Meliț, L.E.; Cucuiet, M.T.; Cucuiet, M.; Rațiu, M.; Săsăran, M.O. COVID-19 Vaccine—A Potential Trigger for MOGAD Transverse Myelitis in a Teenager—A Case Report and a Review of the Literature. Children 2022, 9, 674. https://doi.org/10.3390/children9050674
Mărginean CO, Meliț LE, Cucuiet MT, Cucuiet M, Rațiu M, Săsăran MO. COVID-19 Vaccine—A Potential Trigger for MOGAD Transverse Myelitis in a Teenager—A Case Report and a Review of the Literature. Children. 2022; 9(5):674. https://doi.org/10.3390/children9050674
Chicago/Turabian StyleMărginean, Cristina Oana, Lorena Elena Meliț, Maria Teodora Cucuiet, Monica Cucuiet, Mihaela Rațiu, and Maria Oana Săsăran. 2022. "COVID-19 Vaccine—A Potential Trigger for MOGAD Transverse Myelitis in a Teenager—A Case Report and a Review of the Literature" Children 9, no. 5: 674. https://doi.org/10.3390/children9050674
APA StyleMărginean, C. O., Meliț, L. E., Cucuiet, M. T., Cucuiet, M., Rațiu, M., & Săsăran, M. O. (2022). COVID-19 Vaccine—A Potential Trigger for MOGAD Transverse Myelitis in a Teenager—A Case Report and a Review of the Literature. Children, 9(5), 674. https://doi.org/10.3390/children9050674






