Percutaneous Anorectoplasty (PARP)—An Adaptable, Minimal-Invasive Technique for Anorectal Malformation Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Operative Technique
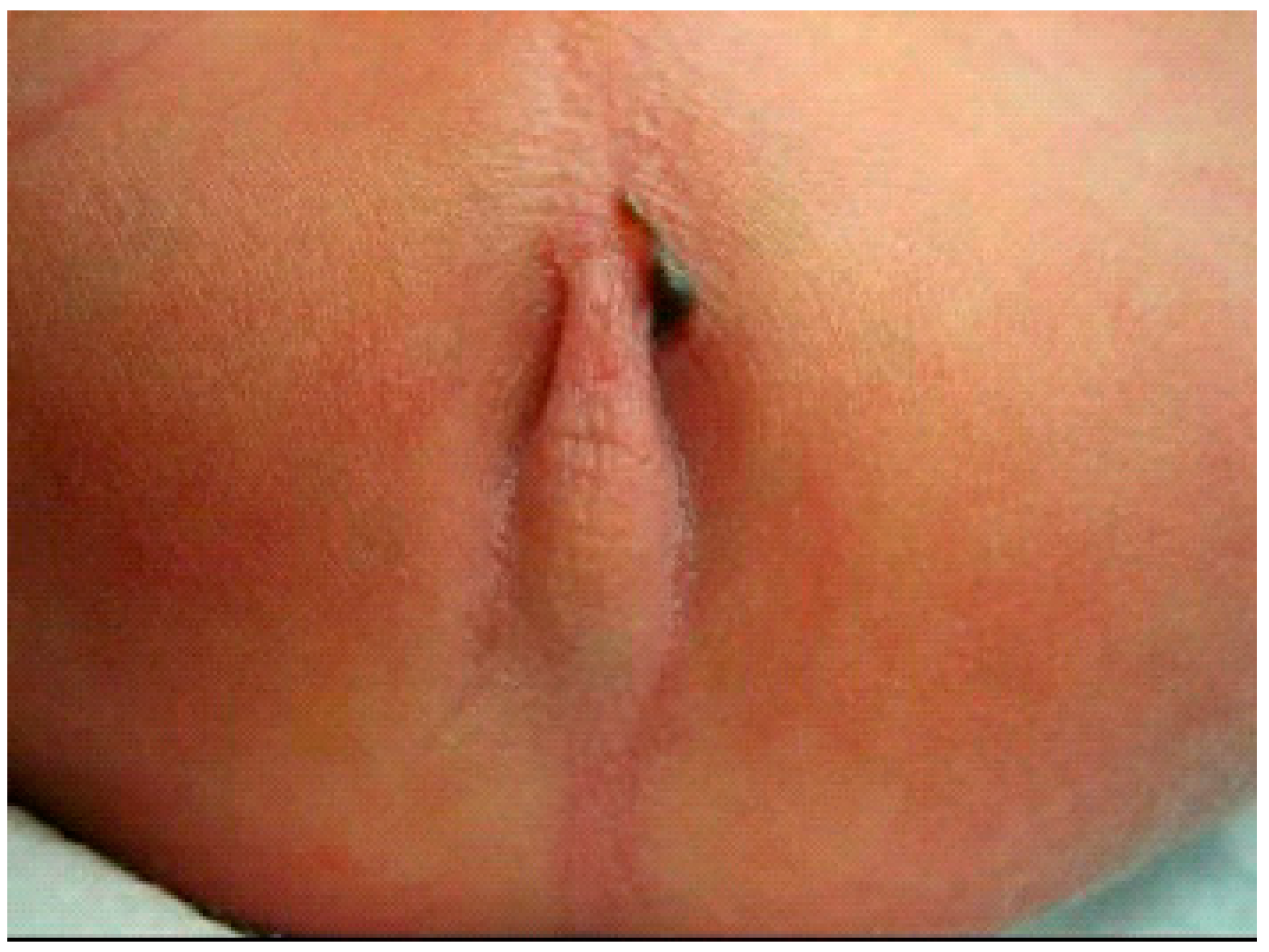
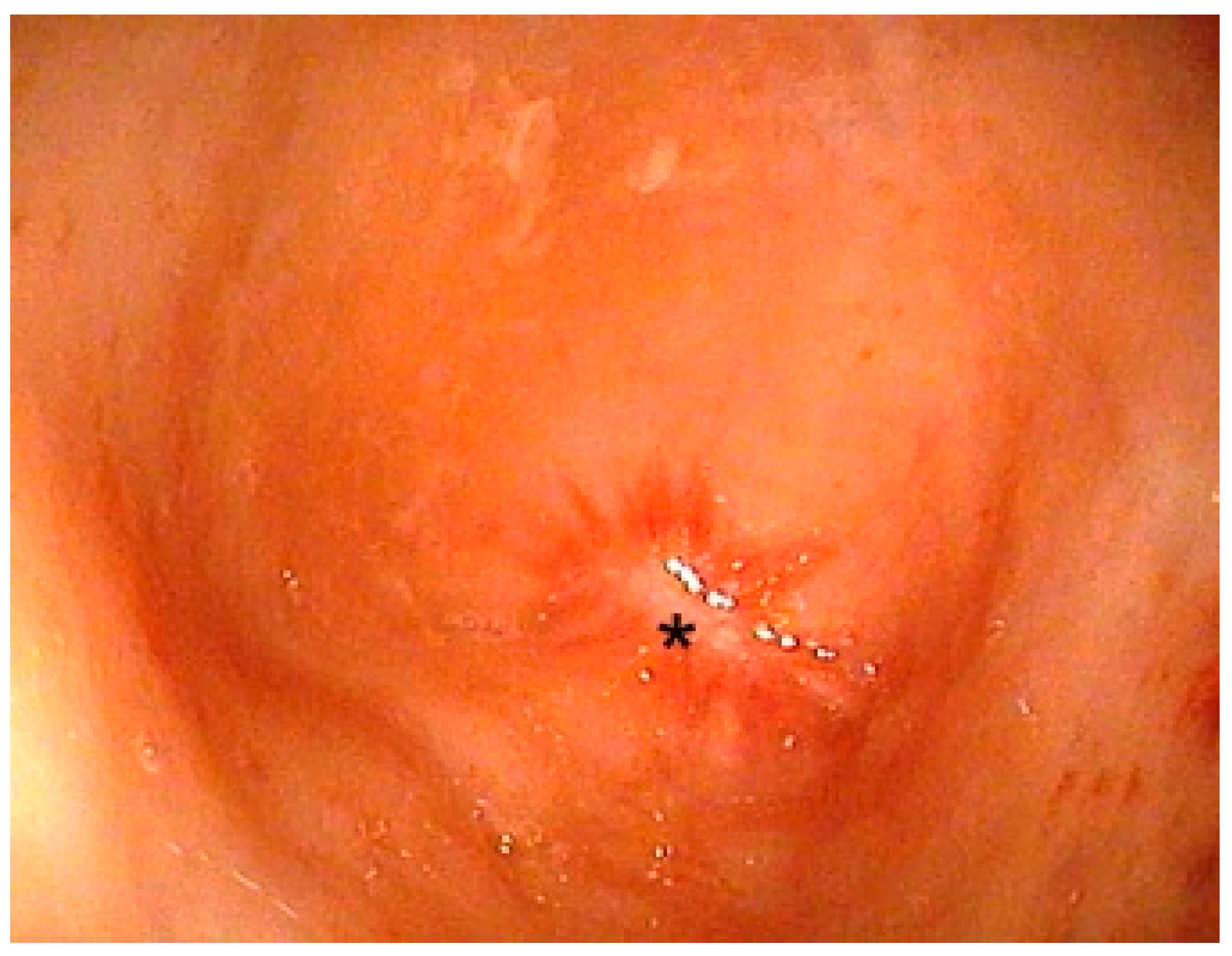
2.3.1. PARP without Image Guidance (nPARP)
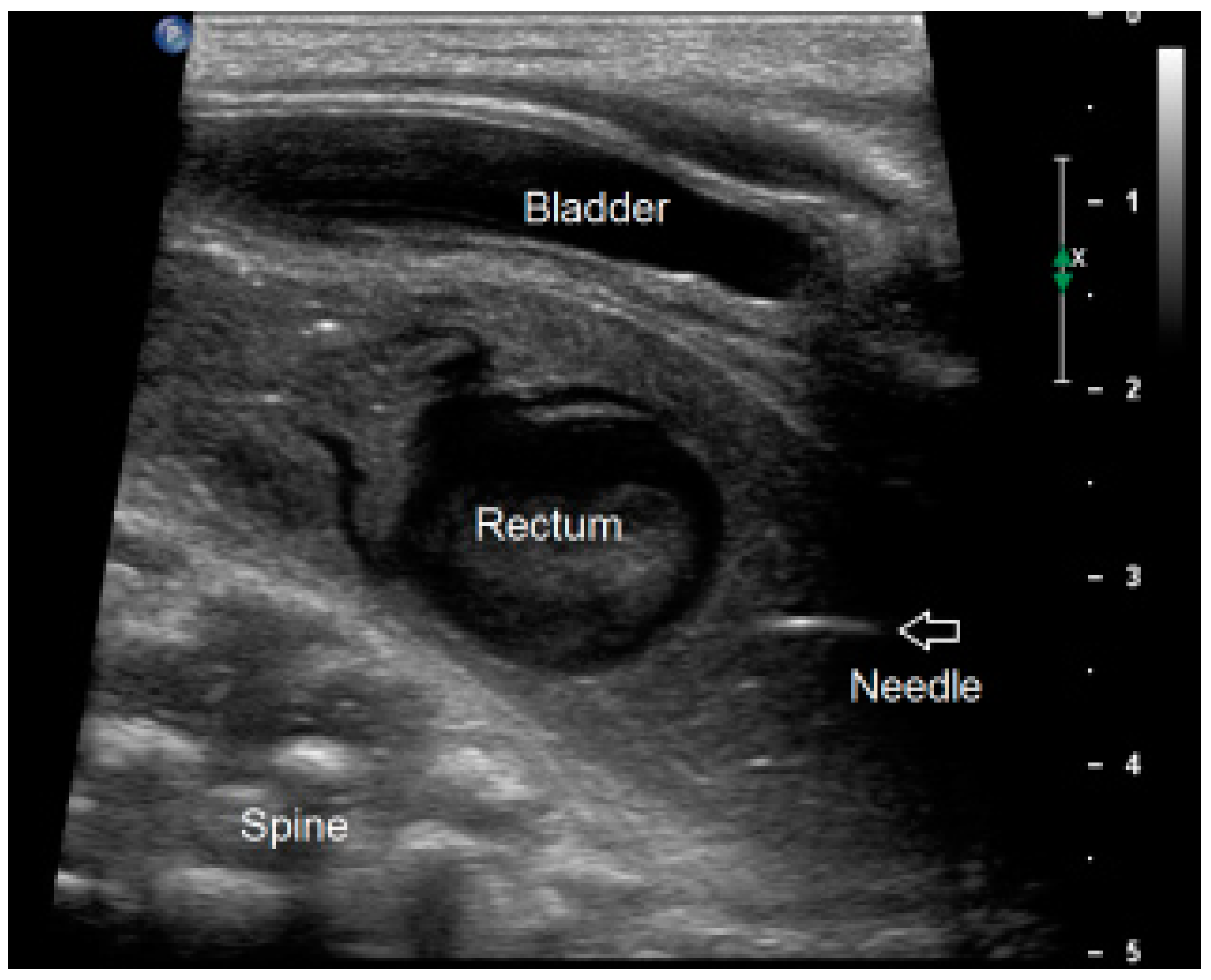
2.3.2. Ultrasound-Guided PARP (uPARP)
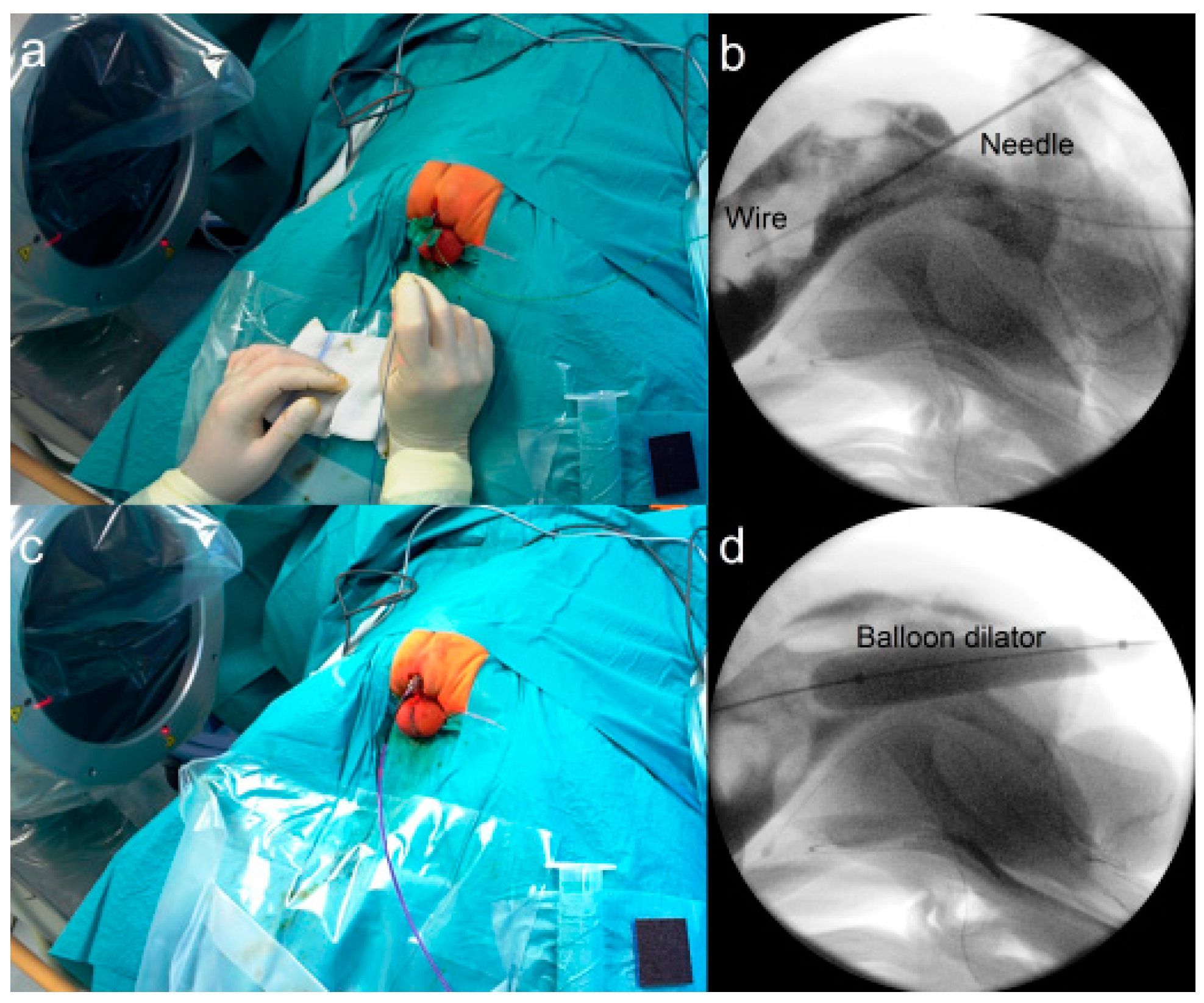
2.3.3. Fluoroscopy-Guided (Interventional) PARP (iPARP)
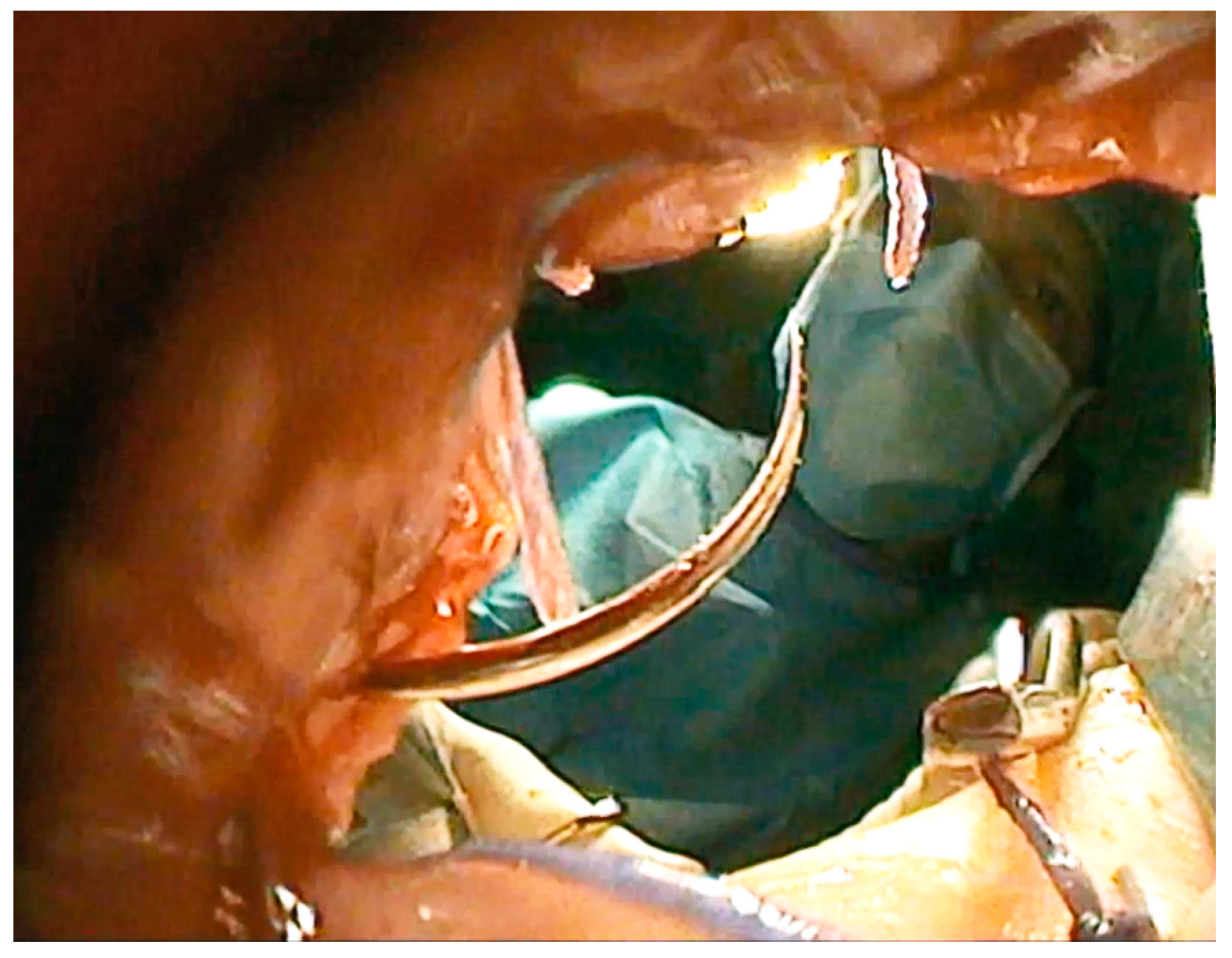
2.3.4. Endoscopically-Guided PARP (ePARP)
2.4. Data Acquisition
3. Results
3.1. Patients
3.2. Operations
3.3. Complications
3.4. Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levitt, M.A.; Peña, A. Anorectal malformations. Fundam. Pediatr. Surg. 2011, 2, 499–512. [Google Scholar]
- Pakarinen, M.P.; Rintala, R.J. Management and outcome of low anorectal malformations. Pediatr. Surg. Int. 2010, 26, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Tofft, L.; Salö, M.; Arnbjörnsson, E.; Stenström, P. Wound dehiscence after posterior sagittal anorectoplasty in children with anorectal malformations. BioMed Res. Int. 2018, 2018, 2930783. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.C.; User, I.R.; Akcaer, V.; Ceylan, H.; Ozokutan, B.H. Posterior sagittal anorectoplasty in vestibular fistula: With or without colostomy. Pediatr. Surg. Int. 2017, 33, 755–759. [Google Scholar] [CrossRef]
- Divarci, E.; Ergun, O. General complications after surgery for anorectal malformations. Pediatr. Surg. Int. 2020, 36, 431–445. [Google Scholar] [CrossRef]
- Ishimaru, T.; Kawashima, H.; Hayashi, K.; Omata, K.; Sanmoto, Y.; Inoue, M. Laparoscopically assisted anorectoplasty—Surgical procedures and outcomes: A literature review. Asian J. Endosc. Surg. 2021, 14, 335–345. [Google Scholar] [CrossRef]
- Georgeson, K.E.; Inge, T.H.; Albanese, C.T. Laparoscopically assisted anorectal pull-through for high imperforate anus—A new technique. J. Pediatr. Surg. 2000, 35, 927–931. [Google Scholar] [CrossRef]
- Morandi, A.; Ure, B.; Leva, E.; Lacher, M. Survey on the management of anorectal malformations (ARM) in European pediatric surgical centers of excellence. Pediatr. Surg. Int. 2015, 31, 543–550. [Google Scholar] [CrossRef]
- van Der Steeg, H.; van Rooij, I.; Iacobelli, B.; Sloots, C.E.J.; Leva, E.; Broens, P.; Leon, F.F.; Makedonsky, I.; Schmiedeke, E.; Vázquez, A.G.; et al. The impact of perioperative care on complications and short term outcome in ARM type rectovestibular fistula: An ARM-Net consortium study. J. Pediatr. Surg. 2019, 54, 1595–1600. [Google Scholar] [CrossRef]
- Han, Y.; Xia, Z.; Guo, S.; Yu, X.; Li, Z. Laparoscopically assisted anorectal pull-through versus posterior sagittal anorectoplasty for high and intermediate anorectal malformations: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0170421. [Google Scholar] [CrossRef]
- Pakarinen, M.P.; Baillie, C.; Koivusalo, A.; Rintala, R.J. Transanal endoscopic-assisted proctoplasty—A novel surgical approach for individual management of patients with imperforate anus without fistula. J. Pediatr. Surg. 2006, 41, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Cairo, S.B.; Rothstein, D.H.; Harmon, C.M. Minimally invasive surgery in the management of anorectal malformations. Clin. Perinatol. 2017, 44, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Rentea, R.M.; Halleran, D.R.; Wood, R.J.; Levitt, M.A. The role of laparoscopy in anorectal malformations. Eur. J. Pediatr. Surg. 2020, 30, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.M.; Abou Zeid, A.A.; El Shafei, I.; Ghanem, W.; Albaghdady, A. Repair of low anorectal anomalies in female patients: Risk factors for wound dehiscence. Ann. Pediatr. Surg. 2017, 13, 140–144. [Google Scholar] [CrossRef]
- Martynov, I.; Gosemann, J.-H.; Hofmann, A.D.; Kuebler, J.F.; Madadi-Sanjani, O.; Ure, B.M.; Lacher, M. Vacuum-assisted closure (VAC) prevents wound dehiscence following posterior sagittal anorectoplasty (PSARP): An exploratory case–control study. J. Pediatr. Surg. 2021, 56, 745–749. [Google Scholar] [CrossRef]
- Kuijper, C.F.; Aronson, D.C. Anterior or posterior sagittal anorectoplasty without colostomy for low-type anorectal malformation: How to get a better outcome? J. Pediatr. Surg. 2010, 45, 1505–1508. [Google Scholar] [CrossRef]
- Ohman, K.A.; Wan, L.; Guthrie, T.; Johnston, B.; Leinicke, J.A.; Glasgow, S.C.; Hunt, S.R.; Mutch, M.G.; Wise, P.E.; Silviera, M.L. Combination of oral antibiotics and mechanical bowel preparation reduces surgical site infection in colorectal surgery. J. Am. Coll. Surg. 2017, 225, 465–471. [Google Scholar] [CrossRef]
- Okada, A.; Kamata, S.; Imura, K.; Fukuzawa, M.; Kubota, A.; Yagi, M.; Azuma, T.; Tsuji, H. Anterior sagittal anorectoplasty for rectovestibular and anovestibular fistula. J. Pediatr. Surg. 1992, 27, 85–88. [Google Scholar] [CrossRef]
- Bischoff, A.; Levitt, M.A.; Peña, A. Update on the management of anorectal malformations. Pediatr. Surg. Int. 2013, 29, 899–904. [Google Scholar] [CrossRef]
- Pakarinen, M.P.; Goyal, A.; Koivusalo, A.; Baillie, C.; Turnock, R.; Rintala, R.J. Functional outcome in correction of perineal fistula in boys with anoplasty versus posterior sagittal anorectoplasty. Pediatr. Surg. Int. 2006, 22, 961–965. [Google Scholar] [CrossRef]
- Peña, A.; Migotto-Krieger, M.; Levitt, M.A. Colostomy in anorectal malformations: A procedure with serious but preventable complications. J. Pediatr. Surg. 2006, 41, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, J.; Geng, J.; Wang, C.; Li, T. The treatment of high and intermediate anorectal malformations: One stage or three procedures? J. Pediatr. Surg. 2004, 39, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Albanese, C.T.; Jennings, R.W.; Lopoo, J.B.; Bratton, B.J.; Harrison, M.R. One-stage correction of high imperforate anus in the male neonate. J. Pediatr. Surg. 1999, 34, 834–836. [Google Scholar] [CrossRef]
- Chan, K.W.E.; Lee, K.H.; Wong, H.Y.V.; Tsui, S.Y.B.; Wong, Y.S.; Pang, K.Y.K.; Mou, J.W.C.; Tam, Y.H. Outcome of patients after single-stage repair of perineal fistula without colostomy according to the Krickenbeck classification. J. Pediatr. Surg. 2014, 49, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Rintala, R.J.; Mildh, L.; Lindahl, H. H-type anorectal malformations: Incidence and clinical characteristics. J. Pediatr. Surg. 1996, 31, 559–562. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.K. Diversities of H-type anorectal malformation: A systematic review on a rare variant of the Krickenbeck classification. Pediatr. Surg. Int. 2017, 33, 3–13. [Google Scholar] [CrossRef]
- Hong, A.R.; Acu, M.F.; Pe, A.; Chaves, L.; Rodriguez, G. Urologic injuries associated with repair of anorectal malformations in male patients. J. Pediatr. Surg. 2002, 37, 339–344. [Google Scholar] [CrossRef]
- Pakarinen, M.P.; Koivusalo, A.; Lindahl, H.; Rintala, R. Prospective controlled long-term follow-up for functional outcome after anoplasty in boys with perineal fistula. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 436–439. [Google Scholar] [CrossRef]
| Patient | Sex | Year | Type of Anorectal Malformation | Comorbidities | Colostomy | Age (Days) at Colostomy |
|---|---|---|---|---|---|---|
| 1 | Male | 2008 | Perineal fistula, bucket handle | None | No | - |
| 2 | Male | 2010 | Perineal fistula (pinpoint at raphe) | None | No | - |
| 3 | Female | 2011 | No fistula | Down syndrome | No | - |
| 4 | Male | 2011 | Perineal fistula | 32-week prematurity, left pneumo-thorax and chest tube placement | No | - |
| 5 | Male | 2015 | Perineal (scrotal) fistula | 36-week prematurity, diagnosed later with Duchenne | No | - |
| 6 | Male | 2015 | Perineal fistula | Currarino triad, Spina bifida, congenital heart disease | No | - |
| 7 | Female | 2015 | No fistula | Down syndrom | Yes | 3 |
| 8 | Male | 2017 | No fistula | None | Yes | 3 |
| 9 | Male | 2020 | No fistula | Down Syndrome | Yes | 1 |
| 10 | Male | 2021 | No fistula | VACTERL association | Yes | 2 |
| Patient | Age at PARP (Days) | Operative Time (Minutes) | Type of PARP | Intraoperative and Perioperative Omplications |
|---|---|---|---|---|
| 1 | 2 | 25 | No image guidance | None |
| 2 | 2 | 183 | No image guidance | Injured urethra or second fistula, converted to psarp, urethra repaired, colostomy performed |
| 3 | 3 | 68 | uPARP | None |
| 4 | 1 | 57 | iPARP | None |
| 5 | 1 | 109 | iPARP | None |
| 6 | 3 | 44 | iPARP | None |
| 7 | 225 | 51 | ePARP | None |
| 8 | 77 | 62 | ePARP | None |
| 9 | 168 | 236 * | ePARP | None |
| 10 | 311 | 111 § | ePARP | None |
| Patient | Age at Last Follow-Up | Constipation | Incontinence | Dilations | Additional Comments |
|---|---|---|---|---|---|
| 1 | 2 y 3 m | No | No | No | Potty trained at 2 years, functionally normal |
| 2 | 2 m | - | - | Yes | Short-term well, long-term lost to follow-up |
| 3 | 1 y 3 m | Yes | No | Yes | Needed macrogol, otherwise no problems in the follow-up time period |
| 4 | 1 y 8 m | No | No | No | Started potty training |
| 5 | 3 y 9 m | No | No | No | No problems, normal stooling pattern, general hypotonia due to duchenne muscular dystrophy in toddlerhood |
| 6 | 6 m | No | No | Yes | Died of congenital heart disease at 6 months |
| 7 | 2 y 10 m | No | No | No | Colostomy takedown at 9 months of age, normal spontaneous defecation pattern 1× per day |
| 8 | 9 m | No | No | No | Colostomy performed at the umbilicus, no issues with stooling, no medications |
| 9 | 1 y 11 m | No | No | No | Started potty training |
| 10 | 8 m | Yes | No | Yes | Too early to evaluate continence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küppers, J.; van Eckert, V.; Muensterer, N.R.; Holler, A.-S.; Rohleder, S.; Kawano, T.; Gödeke, J.; Muensterer, O.J. Percutaneous Anorectoplasty (PARP)—An Adaptable, Minimal-Invasive Technique for Anorectal Malformation Repair. Children 2022, 9, 587. https://doi.org/10.3390/children9050587
Küppers J, van Eckert V, Muensterer NR, Holler A-S, Rohleder S, Kawano T, Gödeke J, Muensterer OJ. Percutaneous Anorectoplasty (PARP)—An Adaptable, Minimal-Invasive Technique for Anorectal Malformation Repair. Children. 2022; 9(5):587. https://doi.org/10.3390/children9050587
Chicago/Turabian StyleKüppers, Julia, Viviane van Eckert, Nadine R. Muensterer, Anne-Sophie Holler, Stephan Rohleder, Takafumi Kawano, Jan Gödeke, and Oliver J. Muensterer. 2022. "Percutaneous Anorectoplasty (PARP)—An Adaptable, Minimal-Invasive Technique for Anorectal Malformation Repair" Children 9, no. 5: 587. https://doi.org/10.3390/children9050587
APA StyleKüppers, J., van Eckert, V., Muensterer, N. R., Holler, A.-S., Rohleder, S., Kawano, T., Gödeke, J., & Muensterer, O. J. (2022). Percutaneous Anorectoplasty (PARP)—An Adaptable, Minimal-Invasive Technique for Anorectal Malformation Repair. Children, 9(5), 587. https://doi.org/10.3390/children9050587







