Subphenotypes in Non-Syndromic Orofacial Cleft Patients Based on the Tooth Agenesis Code (TAC)
Abstract
1. Introduction
2. Materials and Methods
- –
- Non-syndromic orofacial cleft Caucasian patients;
- –
- Patients older than 8 years old so that agenesis of the second premolars could not be mistakenly documented;
- –
- Complete records including charts; photos; panoramic radiographs; and dental casts;
- –
- No history of prior orthodontic treatment or extraction of permanent teeth;
- –
- No presurgical dentofacial orthopedics, gingivoperiosteoplasty or primary bone grafting so that the tooth agenesis presented could not be regarded as iatrogenic;
- –
- All patient records were taken before the secondary alveolar bone grafting;
- –
- The third molars were not assessed in our investigation.
Statistical Analysis
3. Results
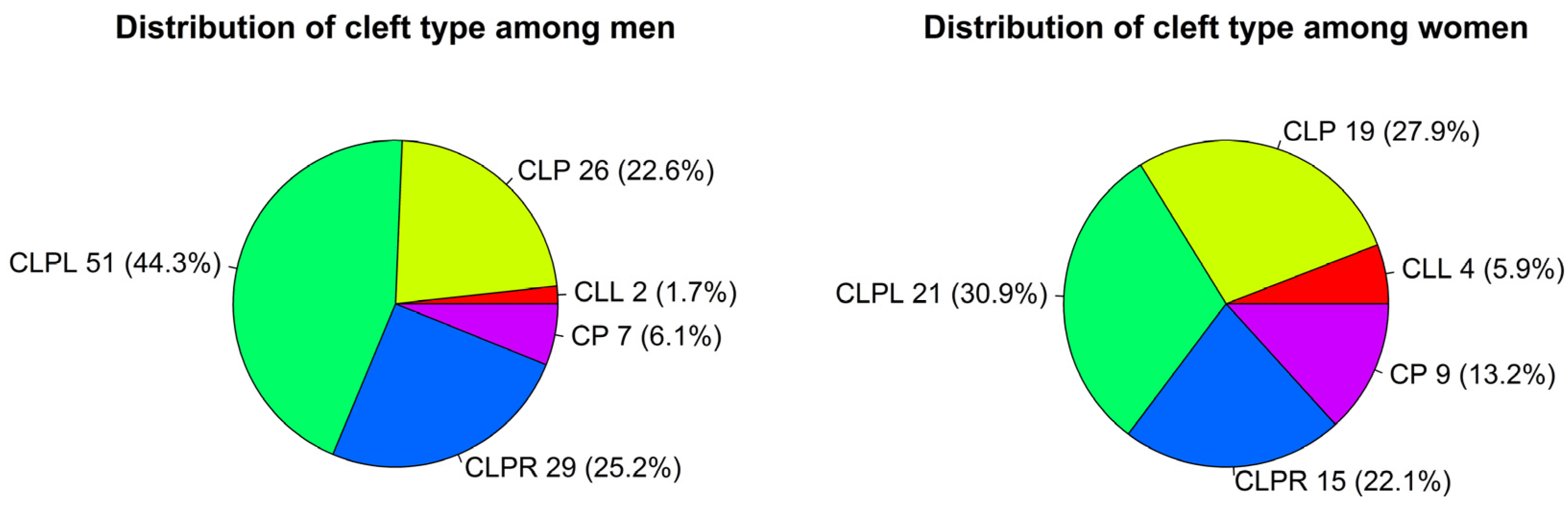
3.1. Cleft Distribution and Association with Sex
3.2. TAC Distribution and Association with Cleft Type and Sex
3.3. Tooth Agenesis and Association with Cleft Type and Sex
3.4. Inter-Quadrant Association
3.5. Odds Ratios (95% CI and p) for the Association of Tooth Agenesis (at Least One Tooth Being Missing) with Gender and Cleft Type
3.6. Most Frequently Missing Teeth by Cleft Type
3.7. Error of the Study
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanier, P.; Moore, G.E. Genetics of cleft lip and palate: Syndromic genes contribute to the incidence of non-syndromic clefts. Hum. Mol. Genet. 2004, 13, R73–R81. [Google Scholar] [CrossRef] [PubMed]
- Seto-Salvia, N.; Stanier, P. Genetics of cleft lip and/or cleft palate: Association with other common anomalies. Eur. J. Med. Genet. 2014, 57, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Heydari, M.; Bokaee, S.; Darvishi, F.; Mohammadi, M. Global prevalence of cleft palate, cleft lip and cleft palate and lip: A comprehensive systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Al Jamal, G.A.; Hazza’a, A.M.; Rawashdeh, M.A. Prevalence of dental anomalies in a population of cleft lip and palate patients. Cleft Palate-Craniofac. J. 2010, 47, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Konstantonis, D.; Alexandropoulos, A.; Konstantoni, N.; Nassika, M. A cross-sectional analysis of the prevalence of tooth agenesis and structural dental anomalies in association with cleft type in non-syndromic oral cleft patients. Prog. Orthod. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Paranaiba, L.M.; Coletta, R.D.; Swerts, M.S.; Quintino, R.P.; de Barros, L.M.; Martelli-Junior, H. Prevalence of Dental Anomalies in Patients With Nonsyndromic Cleft Lip and/or Palate in a Brazilian Population. Cleft Palate-Craniofac. J. 2013, 50, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Wangsrimongkol, T.; Manosudprasit, M.; Pisek, P.; Chittiwatanapong, N. Prevalence and types of dental anomaly in a Thai non-syndromic oral cleft sample. J. Med. Assoc. Thai 2013, 96 (Suppl. 4), S25–S35. [Google Scholar]
- Bartzela, T.N.; Carels, C.E.; Bronkhorst, E.M.; Kuijpers-Jagtman, A.M. Tooth agenesis patterns in unilateral cleft lip and palate in humans. Arch. Oral Biol. 2013, 58, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gimenez, A.; Silvestre-Rangil, J.; Silvestre, F.J.; Paredes-Gallardo, V. Tooth agenesis code (TAC) in complete unilateral and bilateral cleft lip and palate patients. Odontology 2018, 106, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Lubit, E.; Kuftinec, M.M. Congenitally missing second premolars in cleft lip and cleft palate children. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 396–400. [Google Scholar] [CrossRef]
- Halpern, R.M.; Noble, J. Location and presence of permanent teeth in a complete bilateral cleft lip and palate population. Angle Orthod. 2010, 80, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Vastardis, H. The genetics of human tooth agenesis: New discoveries for understanding dental anomalies. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 650–656. [Google Scholar] [CrossRef]
- Van den Boogaard, M.J.; Dorland, M.; Beemer, F.A.; van Amstel, H.K. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat. Genet. 2000, 24, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Park, J.W.; Kim, Y.H.; Baek, S.H. Associations between the risk of tooth agenesis and single-nucleotide polymorphisms of MSX1 and PAX9 genes in nonsyndromic cleft patients. Angle Orthod 2013, 83, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, A.M.; Luijsterburg, A.J.; Ongkosuwito, E.M.; van den Boogaard, M.J.; de Vries, E.; Hovius, S.E.; Vermeij-Keers, C. Delayed diagnosis and underreporting of congenital anomalies associated with oral clefts in the Netherlands: A national validation study. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Modesto, A.; Meira, R.; Barbosa, A.R.; Lidral, A.C.; Murray, J.C. Interferon regulatory factor 6 (IRF6) and fibroblast growth factor receptor 1 (FGFR1) contribute to human tooth agenesis. Am. J. Med. Genet. A 2007, 143A, 538–545. [Google Scholar] [CrossRef]
- Basha, M.; Demeer, B.; Revencu, N.; Helaers, R.; Theys, S.; Bou Saba, S.; Boute, O.; Devauchelle, B.; Francois, G.; Bayet, B.; et al. Whole exome sequencing identifies mutations in 10% of patients with familial non-syndromic cleft lip and/or palate in genes mutated in well-known syndromes. J. Med. Genet. 2018, 55, 449–458. [Google Scholar] [CrossRef]
- van Wijk, A.J.; Tan, S.P. A numeric code for identifying patterns of human tooth agenesis: A new approach. Eur. J. Oral Sci. 2006, 114, 97–101. [Google Scholar] [CrossRef]
- Hermus, R.R.; van Wijk, A.J.; Tan, S.P.; Kramer, G.J.; Ongkosuwito, E.M. Patterns of tooth agenesis in patients with orofacial clefts. Eur. J. Oral Sci. 2013, 121, 328–332. [Google Scholar] [CrossRef]
- Bartzela, T.N.; Carels, C.E.; Bronkhorst, E.M.; Ronning, E.; Rizell, S.; Kuijpers-Jagtman, A.M. Tooth agenesis patterns in bilateral cleft lip and palate. Eur. J. Oral Sci. 2010, 118, 47–52. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Vieira, A.R. Oral clefts and syndromic forms of tooth agenesis as models for genetics of isolated tooth agenesis. J. Dent. Res. 2003, 82, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wong, S.W.; Han, D.; Cai, T. Genetic analysis: Wnt and other pathways in nonsyndromic tooth agenesis. Oral Dis. 2019, 25, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Mink van der Molen, A.B.; van Breugel, J.M.M.; Janssen, N.G.; Admiraal, R.J.C.; van Adrichem, L.N.A.; Bierenbroodspot, F.; Bittermann, D.; van den Boogaard, M.H.; Broos, P.H.; Dijkstra-Putkamer, J.J.M.; et al. Clinical Practice Guidelines on the Treatment of Patients with Cleft Lip, Alveolus, and Palate: An Executive Summary. J. Clin. Med. 2021, 10, 4813. [Google Scholar] [CrossRef] [PubMed]
- Dewinter, G.; Quirynen, M.; Heidbuchel, K.; Verdonck, A.; Willems, G.; Carels, C. Dental abnormalities, bone graft quality, and periodontal conditions in patients with unilateral cleft lip and palate at different phases of orthodontic treatment. Cleft Palate-Craniofac. J. 2003, 40, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lourenco Ribeiro, L.; Teixeira Das Neves, L.; Costa, B.; Ribeiro Gomide, M. Dental anomalies of the permanent lateral incisors and prevalence of hypodontia outside the cleft area in complete unilateral cleft lip and palate. Cleft Palate-Craniofac. J. 2003, 40, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Chen, P.K.; Lo, L.J.; Cheng, M.C.; Ko, E.W. The characteristics and distribution of dental anomalies in patients with cleft. Chang Gung Med. J. 2011, 34, 306–314. [Google Scholar] [PubMed]
- Khalaf, K.; Miskelly, J.; Voge, E.; Macfarlane, T.V. Prevalence of hypodontia and associated factors: A systematic review and meta-analysis. J. Orthod. 2014, 41, 299–316. [Google Scholar] [CrossRef]
- Polder, B.J.; Van’t Hof, M.A.; Van der Linden, F.P.; Kuijpers-Jagtman, A.M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent. Oral Epidemiol. 2004, 32, 217–226. [Google Scholar] [CrossRef]
- Lagana, G.; Venza, N.; Borzabadi-Farahani, A.; Fabi, F.; Danesi, C.; Cozza, P. Dental anomalies: Prevalence and associations between them in a large sample of non-orthodontic subjects, a cross-sectional study. BMC Oral Health 2017, 17, 62. [Google Scholar] [CrossRef]
- Camporesi, M.; Baccetti, T.; Marinelli, A.; Defraia, E.; Franchi, L. Maxillary dental anomalies in children with cleft lip and palate: A controlled study. Int. J. Paediatr. Dent. 2010, 20, 442–450. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Oginski, T.; Gedrange, T.; Berniczei-Royko, A.; Prussak, E. Prevalence of second premolar hypodontia in the Polish cleft lip and palate population. Med. Sci. Monit. 2014, 20, 355–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beames, T.G.; Lipinski, R.J. Gene-environment interactions: Aligning birth defects research with complex etiology. Development 2020, 147, dev191064. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kouskoura, T.; Fragou, N.; Alexiou, M.; John, N.; Sommer, L.; Graf, D.; Katsaros, C.; Mitsiadis, T.A. The genetic basis of craniofacial and dental abnormalities. Schweiz. Monatsschr. Zahnmed. 2011, 121, 636–646. [Google Scholar] [PubMed]
- Jia, S.; Zhou, J.; Fanelli, C.; Wee, Y.; Bonds, J.; Schneider, P.; Mues, G.; D’Souza, R.N. Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero. Development 2017, 144, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Cocos, A.; Halazonetis, D.J. Craniofacial shape differs in patients with tooth agenesis: Geometric morphometric analysis. Eur. J. Orthod. 2017, 39, 345–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leslie, E.J. Genetic models and approaches to study orofacial clefts. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]


| Upper Right Quadrant (q1) | Upper Left Quadrant (q2) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 17 | 16 | 15 | 14 | 13 | 12 | 11 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| B | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
| A | 47 | 46 | 45 | 44 | 43 | 42 | 41 | 31 | 32 | 33 | 34 | 35 | 36 | 37 |
| Lower Right Quadrant (q4) | Lower Left Quadrant (q3) | |||||||||||||
| Overall (N = 183) | |||
|---|---|---|---|
| Tooth Agenesis Code (TAC) | Tooth/Teeth Missing | N | |
| 000.000.000.000 | 87 (47.5%) | None | 0 |
| 000.002.000.000 | 30 (16.4%) | 22 | 1 |
| 002.000.000.000 | 18 (9.8%) | 12 | 1 |
| 002.002.000.000 | 13 (7.1%) | 12, 22 | 2 |
| 000.000.016.016 | 3 (1.6%) | 35, 45 | 2 |
| 000.000.000.016 | 2 (1.1%) | 45 | 1 |
| 000.016.000.000 | 2 (1.1%) | 25 | 1 |
| 001.000.000.000 | 2 (1.1%) | 11 | 1 |
| 002.001.000.000 | 2 (1.1%) | 12, 21 | 2 |
| 016.000.000.000 | 2 (1.1%) | 15 | 1 |
| 018.000.000.000 | 2 (1.1%) | 12, 15 | 2 |
| 000.000.000.002 | 1 (0.5%) | 42 | 1 |
| 000.000.008.008 | 1 (0.5%) | 34, 44 | 2 |
| 000.000.016.000 | 1 (0.5%) | 35 | 1 |
| 000.003.000.000 | 1 (0.5%) | 21, 22 | 2 |
| 000.006.000.000 | 1 (0.5%) | 22, 23 | 2 |
| 000.010.000.000 | 1 (0.5%) | 22, 24 | 2 |
| 002.000.016.000 | 1 (0.5%) | 12, 35 | 2 |
| 002.002.000.001 | 1 (0.5%) | 12, 22, 41 | 3 |
| 002.010.000.000 | 1 (0.5%) | 12, 22, 24 | 3 |
| 002.018.000.016 | 1 (0.5%) | 12, 22, 25, 45 | 4 |
| 003.000.000.000 | 1 (0.5%) | 11, 12 | 2 |
| 003.002.000.000 | 1 (0.5%) | 11, 12, 22 | 3 |
| 016.002.000.000 | 1 (0.5%) | 15, 22 | 2 |
| 016.002.016.000 | 1 (0.5%) | 15, 22, 35 | 3 |
| 018.002.016.016 | 1 (0.5%) | 12, 15, 22, 35, 45 | 5 |
| 018.016.016.016 | 1 (0.5%) | 12, 15, 25, 35, 45 | 5 |
| 024.024.000.000 | 1 (0.5%) | 14, 15, 24, 25 | 4 |
| 024.024.008.008 | 1 (0.5%) | 14, 15, 24, 25, 34, 44 | 6 |
| 030.027.016.016 | 1 (0.5%) | 12, 13, 14, 15, 21, 22, 24, 25, 35, 45 | 10 |
| 080.080.082.002 | 1 (0.5%) | 15, 17, 25, 27, 32, 35, 37, 42 | 8 |
| 000.000.000.000 (N = 87) | 000.002.000.000 (N = 30) | 002.000.000.000 (N = 18) | 002.002.000.000 (N = 13) | Other (N = 35) | p Value | |
|---|---|---|---|---|---|---|
| Sex | 0.405 * | |||||
| Women | 38 (55.9%) | 8 (11.8%) | 6 (8.8%) | 3 (4.4%) | 13 (19.1%) | |
| Men | 49 (42.6%) | 22 (19.1%) | 12 (10.4%) | 10 (8.7%) | 22 (19.1%) | |
| Orofacial cleft types | 0.001 * | |||||
| CLL | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| CLP | 19 (42.2%) | 6 (13.3%) | 3 (6.7%) | 6 (13.3%) | 11 (24.4%) | |
| CLPL | 34 (47.2%) | 21 (29.2%) | 3 (4.2%) | 4 (5.6%) | 10 (13.9%) | |
| CLPR | 19 (43.2%) | 2 (4.5%) | 12 (27.3%) | 2 (4.5%) | 9 (20.5%) | |
| CP | 9 (56.2%) | 1 (6.2%) | 0 (0.0%) | 1 (6.2%) | 5 (31.2%) |
| Q1 and Q2 | Q2 | p-Value | |||
|---|---|---|---|---|---|
| No | Yes | 0.002 | |||
| Q1 | No | 95 (73.1%) | 35 (26.9%) | ||
| Yes | 26 (49.1%) | 27 (50.9%) | |||
| Q1 and Q3 | Q3 | ||||
| No | Yes | 0.020 | |||
| Q1 | No | 125 (96.2%) | 5 (3.8%) | ||
| Yes | 46 (86.8%) | 7 (13.2%) | |||
| Q1 and Q4 | Q4 | ||||
| No | Yes | 0.071 | |||
| Q1 | No | 123 (94.6%) | 7 (5.4%) | ||
| Yes | 46 (86.8%) | 7 (13.2%) | |||
| Q2 and Q3 | Q3 | ||||
| No | Yes | 0.222 | |||
| Q2 | No | 115 (95.0%) | 6 (5.0%) | ||
| Yes | 56 (90.3%) | 6 (9.7%) | |||
| Q2 and Q4 | Q4 | ||||
| No | Yes | 0.185 | |||
| Q2 | No | 114 (94.2%) | 7 (5.8%) | ||
| Yes | 55 (88.7%) | 7 (11.3%) | |||
| Q3 and Q4 | Q4 | ||||
| No | Yes | <0.001 | |||
| Q3 | No | 166 (97.1%) | 5 (2.9%) | ||
| Yes | 3 (25.0%) | 9 (75.0%) |
| Factors | OR | Lower | Upper | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Women | Ref | |||
| Men | 1.59 | 0.85 | 2.99 | 0.149 |
| Cleft type | 0.799 | |||
| CLP | Ref | |||
| CLPL | 0.77 | 0.35 | 1.63 | 0.493 |
| CLPR | 0.93 | 0.39 | 2.16 | 0.857 |
| CP | 0.6 | 0.18 | 1.92 | 0.392 |
| In the first quadrant | ||||
| Sex | ||||
| Women | Ref | |||
| Men | 1.56 | 0.77 | 3.25 | 0.225 |
| Cleft type | 0.022 | |||
| CLP | Ref | |||
| CLPL | 0.37 | 0.16 | 0.87 | 0.023 |
| CLPR | 1.21 | 0.51 | 2.86 | 0.661 |
| CP | 0.4 | 0.08 | 1.48 | 0.201 |
| In the second quadrant | ||||
| Sex | ||||
| Women | Ref | |||
| Men | 1.68 | 0.84 | 3.46 | 0.147 |
| Cleft type | 0.002 | |||
| CLP | Ref | |||
| CLPL | 0.85 | 0.4 | 1.83 | 0.681 |
| CLPR | 0.14 | 0.04 | 0.39 | <0.001 |
| CP | 0.4 | 0.1 | 1.38 | 0.167 |
| In the third quadrant | ||||
| Sex | ||||
| Women | Ref | |||
| Men | 0.69 | 0.2 | 2.45 | 0.555 |
| Cleft type | 0.082 | |||
| CLP | Ref | |||
| CLPL | 0.64 | 0.11 | 3.63 | 0.597 |
| CLPR | 0.69 | 0.09 | 4.37 | 0.69 |
| CP | 4.46 | 0.86 | 25.55 | 0.074 |
| In the fourth quadrant | ||||
| Sex | ||||
| Women | Ref | |||
| Men | 1.35 | 0.42 | 4.89 | 0.621 |
| Cleft type | 0.078 | |||
| CLP | Ref | |||
| CLPL | 0.43 | 0.08 | 2.05 | 0.285 |
| CLPR | 0.73 | 0.14 | 3.53 | 0.696 |
| CP | 3.58 | 0.74 | 17.62 | 0.105 |
| CLP (N = 45) | CLPL (N = 72) | CLPR (N = 44) | CP (N = 16) | Total (N = 183) | p Value | |
|---|---|---|---|---|---|---|
| 11 | 2 (4.4%) | 0 (0.0%) | 2 (4.5%) | 0 (0.0%) | 4 (2.2%) | 0.149 |
| 12 | 15 (33.3%) | 10 (13.9%) | 17 (38.6%) | 2 (12.5%) | 44 (24.0%) | 0.006 |
| 13 | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0.593 |
| 14 | 2 (4.4%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 3 (1.6%) | 0.593 |
| 15 | 4 (8.9%) | 5 (6.9%) | 2 (4.5%) | 1 (6.2%) | 12 (6.6%) | 0.872 |
| 16 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 17 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (0.5%) | 0.090 |
| 21 | 3 (6.7%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 4 (2.2%) | 0.223 |
| 22 | 17 (37.8%) | 30 (41.7%) | 4 (9.1%) | 3 (18.8%) | 54 (29.5%) | <0.001 |
| 23 | 0 (0.0%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 1.000 |
| 24 | 3 (6.7%) | 2 (2.8%) | 0 (0.0%) | 0 (0.0%) | 5 (2.7%) | 0.236 |
| 25 | 4 (8.9%) | 2 (2.8%) | 1 (2.3%) | 1 (6.2%) | 8 (4.4%) | 0.353 |
| 26 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 27 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (0.5%) | 0.090 |
| 31 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 32 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (0.5%) | 0.090 |
| 33 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 34 | 0 (0.0%) | 1 (1.4%) | 1 (2.3%) | 0 (0.0%) | 2 (1.1%) | 0.792 |
| 35 | 3 (6.7%) | 2 (2.8%) | 1 (2.3%) | 4 (25.0%) | 10 (5.5%) | 0.014 |
| 37 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (0.5%) | 0.090 |
| 41 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.2%) | 1 (0.5%) | 0.090 |
| 42 | 0 (0.0%) | 0 (0.0%) | 1 (2.3%) | 1 (6.2%) | 2 (1.1%) | 0.053 |
| 43 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 44 | 0 (0.0%) | 1 (1.4%) | 1 (2.3%) | 0 (0.0%) | 2 (1.1%) | 0.792 |
| 45 | 4 (8.9%) | 2 (2.8%) | 1 (2.3%) | 2 (12.5%) | 9 (4.9%) | 0.166 |
| 47 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantonis, D.; Nassika, M.; Athanasiou, M.; Vastardis, H. Subphenotypes in Non-Syndromic Orofacial Cleft Patients Based on the Tooth Agenesis Code (TAC). Children 2022, 9, 437. https://doi.org/10.3390/children9030437
Konstantonis D, Nassika M, Athanasiou M, Vastardis H. Subphenotypes in Non-Syndromic Orofacial Cleft Patients Based on the Tooth Agenesis Code (TAC). Children. 2022; 9(3):437. https://doi.org/10.3390/children9030437
Chicago/Turabian StyleKonstantonis, Dimitrios, Maria Nassika, Maria Athanasiou, and Heleni Vastardis. 2022. "Subphenotypes in Non-Syndromic Orofacial Cleft Patients Based on the Tooth Agenesis Code (TAC)" Children 9, no. 3: 437. https://doi.org/10.3390/children9030437
APA StyleKonstantonis, D., Nassika, M., Athanasiou, M., & Vastardis, H. (2022). Subphenotypes in Non-Syndromic Orofacial Cleft Patients Based on the Tooth Agenesis Code (TAC). Children, 9(3), 437. https://doi.org/10.3390/children9030437






