The Relation between Vitamin D Level and Lung Clearance Index in Cystic Fibrosis—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Data Collected
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Vitamin D Status
3.3. LCI Variability
3.4. Correlations of 25-OH Vitamin D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balfour-Lynn, I.M.; King, J.A. CFTR modulator therapies—Effect on life expectancy in people with cystic fibrosis. Paediatr. Respir. Rev. 2020, S1526-0542(20)30081-6. [Google Scholar] [CrossRef]
- Dediu, M.; Ciuca, I.M.; Marc, M.S.; Boeriu, E.; Pop, L.L. Factors Influencing Lung Function in Patients with Cystic Fibrosis in Western Romania. J. Multidiscip. Healthc. 2021, 14, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.C.; Hall, G.L.; Sly, P.D.; Stick, S.M.; Douglas, T.A. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF). Early Lung Disease in Infants and Preschool Children with Cystic Fibrosis. What Have We Learned and What Should We Do about It? Am. J. Respir. Crit. Care Med. 2017, 195, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Coverstone, A.M.; Ferkol, T.W. Early Diagnosis and Intervention in Cystic Fibrosis: Imagining the Unimaginable. Front. Pediatr. 2021, 8, 608821. [Google Scholar] [CrossRef] [PubMed]
- Rybacka, A.; Goździk-Spychalska, J.; Rybacki, A.; Piorunek, T.; Batura-Gabryel, H.; Karmelita-Katulska, K. Congruence Between Pulmonary Function and Computed Tomography Imaging Assessment of Cystic Fibrosis Severity. Adv. Exp. Med. Biol. 2018, 1114, 67–76. [Google Scholar] [CrossRef]
- Fretzayas, A.; Loukou, I.; Moustaki, M.; Douros, K. Correlation of computed tomography findings and lung function in children and adolescents with cystic fibrosis. World J. Pediatr. 2021, 17, 221–226. [Google Scholar] [CrossRef]
- Fretzayas, A.; Douros, K.; Moustaki, M.; Loukou, I. Applications of lung clearance index in monitoring children with cystic fibrosis. World J. Clin. Pediatr. 2019, 8, 15–22. [Google Scholar] [CrossRef]
- Kasi, A.S.; Wee, C.P.; Keens, T.G.; Salinas, D.B. Abnormal Lung Clearance Index in Cystic Fibrosis Children with Normal FEV1 and Single-Breath Nitrogen Washout Test. Lung 2021, 199, 37–41. [Google Scholar] [CrossRef]
- Mitri, C.; Xu, Z.; Bardin, P.; Corvol, H.; Touqui, L.; Tabary, O. Novel Anti-Inflammatory Approaches for Cystic Fibrosis Lung Disease: Identification of Molecular Targets and Design of Innovative Therapies. Front. Pharmacol. 2020, 11, 1096. [Google Scholar] [CrossRef]
- Genç, D.; Sezer Kürkçü, M.; Günaydin, B.; Tarhan, E.F. 1,25-dihydroxyvitamin D3 regulates t helper and b lymphocyte responses substantially in drug-naive primary Sjögren’s syndrome patients’ mononuclear cells. Turk. J. Med. Sci. 2021, 51, 2467–2476. [Google Scholar] [CrossRef]
- Ciuca, I.M.; Pop, L.L.; Dediu, M.; Tanasescu, S.A.; Ardelean, F.; Iovanescu, G.; Boeriu, E.; Vlad, C.D.; Marc, M.S.; Almajan, B. Vitamin D(25-OH-cholecalciferol) in Cystic Fibrosis and the Relations with Cholesterol and Proteins. Rev. Chim. 2019, 70, 3185–3187. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [Green Version]
- Nitsa, A.; Toutouza, M.; Machairas, N.; Mariolis, A.; Philippou, A.; Koutsilieris, M. Vitamin D in Cardiovascular Disease. In Vivo 2018, 32, 977–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2018, 65, 74–80. [Google Scholar] [CrossRef]
- Avestaei, A.-H.; Yaghchiyan, M.; Ali-Hemmati, A.; Farhangi, M.A.; Mesgari-Abbasi, M.; Shahabi, P. Histological, metabolic, and inflammatory changes in the renal tissues of high-fat diet-induced obese rats after vitamin D supplementation. Nutr. Food Sci. 2020, 50, 1135–1149. [Google Scholar] [CrossRef]
- Panfili, F.M.; Roversi, M.; D’Argenio, P.; Rossi, P.; Cappa, M.; Fintini, D. Possible role of vitamin D in Covid-19 infection in pediatric population. J. Endocrinol. Investig. 2021, 44, 27–35. [Google Scholar] [CrossRef]
- Wang, T.-T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D 3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [Green Version]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D 3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin, C.; Vitamin, D.; and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Kolls, J.K.; Garry, R.F. Role of the T cell vitamin D receptor in severe COVID-19. Nat. Immunol. 2022, 23, 5–6. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.O.; Pamukcu, E.; Yakar, B. The role of vitamin D deficiency on COVID-19: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2021, 43, e2021074. [Google Scholar] [CrossRef]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Eller-Vainicher, C.; Rossini, M.; Persani, L.; Zambon, A.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 1968. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15. [Google Scholar] [CrossRef] [Green Version]
- Le, T.N. Updates in vitamin D therapy in cystic fibrosis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 361–365. [Google Scholar] [CrossRef]
- Guillien, A.; Soumagne, T.; Regnard, J.; Degano, B.; Groupe Fonction de la SPLF. Les nouvelles équations de référence du Global Lung Function Initiative (GLI) pour les explorations fonctionnelles respiratoires [The new reference equations of the Global Lung function Initiative (GLI) for pulmonary function tests]. Rev. Mal. Respir. 2018, 35, 1020–1027. (In French) [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Hasan, S.; Darukhanavala, A.; Tangpricha, V. A Clinician’s guide to vitamin D supplementation for patients with cystic fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100273. [Google Scholar] [CrossRef]
- Dancey, C.P.; John, R. Statistics without Maths for Psychology: Using Spss for Windows; Prentice-Hall, Inc.: Prentice Hall, UK, 2004. [Google Scholar]
- Tangpricha, V.; Kelly, A.; Stephenson, A.; Maguiness, K.; Enders, J.; Robinson, K.A.; Marshall, B.C.; Borowitz, D.; for the Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: Evidence-based recommendations from the cystic fibrosis foundation. J. Clin. Endocrinol. Metab. 2012, 97, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sánchez, C.; Garriga-García, M.; Serrano-Nieto, M.J.; García-Romero, R.; Álvarez-Beltrán, M.; Crehuá-Gaudiza, E.; Muñoz-Codoceo, R.; Suárez-Cortina, L.; Vicente-Santamaría, S.; Martínez-Costa, C.; et al. Vitamin D Status in Pediatric and Young Adult Cystic Fibrosis Patients. Are the New Recommendations Effective? Nutrients 2021, 13, 4413. [Google Scholar] [CrossRef] [PubMed]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D deficiency and its treatment in cystic fibrosis. J. Cyst. Fibros. 2019, 18, S66–S73. [Google Scholar] [CrossRef] [Green Version]
- Dediu, M.; Ciuca, I.; Pop, L.L. P205 Influences on vitamin D in cystic fibrosis patients. J. Cyst. Fibros. 2018, 17, S117. [Google Scholar] [CrossRef]
- Thursfield, R.M.; Naderi, K.; Leaver, N.; Rosenthal, M.; Alton, E.W.F.W.; Bush, A.; Bush, A.; Davies, J.C. Children with cystic fibrosis demonstrate no respiratory immunological, infective or physiological, consequences of vitamin D deficiency. J. Cyst. Fibros. 2018, 17, 657–665. [Google Scholar] [CrossRef]
- Dediu, M.; Pop, L.L.; Duta, G.; Ciuca, I. P184 Vitamin d correlation with lung function in cystic fibrosis children. Arch. Dis. Child. 2017, 102, A105. [Google Scholar] [CrossRef]
- Lum, S.; Stocks, J.; Stanojevic, S.; Wade, A.; Robinson, P.; Gustafsson, P.; Brown, M.; Aurora, P.; Subbarao, P.; Hoo, A.-F.; et al. Age and height dependence of lung clearance index and functional residual capacity. Eur. Respir. J. 2013, 41, 1371–1377. [Google Scholar] [CrossRef]
- Frauchiger, B.S.; Binggeli, S.; Yammine, S.; Spycher, B.; Krüger, L.; Ramsey, K.A.; Latzin, P. Longitudinal course of clinical lung clearance index in children with cystic fibrosis. Eur. Respir. J. 2021, 58, 2002686. [Google Scholar] [CrossRef]
- Elidottir, H.; Diemer, S.; Eklund, E.; Hansen, C. Abnormal glucose tolerance and lung function in children with cystic fibrosis. Comparing oral glucose tolerance test and continuous glucose monitoring. J. Cyst. Fibros. 2021, 20, 779–784. [Google Scholar] [CrossRef]
- Walicka-Serzysko, K.; Postek, M.; Milczewska, J.; Sands, D. Change in lung clearance index with microbiological status in children with cystic fibrosis. Pediatr. Pulmonol. 2019, 54, 729–736. [Google Scholar] [CrossRef]
- O’Neill, K.; Bradley, J.M.; Reid, A.; Downey, D.G.; Rendall, J.; McCaughan, J.; Moore, J.E.; Tunney, M.M.; Elborn, J.S. Airway infection, systemic inflammation and lung clearance index in children and adults with cystic fibrosis. Eur. Respir. J. 2018, 51, 1701704. [Google Scholar] [CrossRef] [Green Version]
- Abu-Fraiha, Y.; Elyashar-Earon, H.; Shoseyov, D.; Cohen-Cymberknoh, M.; Armoni, S.; Kerem, E.; Wilschanski, M. Increasing Vitamin D Serum Levels Is Associated with Reduced Pulmonary Exacerbations in Patients With Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Nazir, M.; Bhat, J.I.; Malik, E.U.; Ahmad, Q.I.; Charoo, B.A.; Ali, S.W. Vitamin D status correlates with the markers of cystic fibrosis-related pulmonary disease. Pediatr. Neonatol. 2019, 60, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Clinical impact of vitamin D treatment in cystic fibrosis: A pilot randomized, controlled trial. Eur. J. Clin. Nutr. 2017, 71, 203–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| All Patients (N = 56) | 3–6 Years (N = 9) | 6–12 Years (N = 20) | 12–18 Years (N = 17) | 18+ Years (N = 10) | |
|---|---|---|---|---|---|
| Age (years) | 11 (7.00, 15.00) | 5 (4.00, 5.00) | 9 (7.00, 10.00) | 15 (13.50, 15.00) | 19 (10, 20) |
| BMI percentile <25th | 33 (58.9%) | 2 (22.2%) | 9 (45%) | 13 (76.5%) | 9 (90%) |
| CFLD | 32 (57.1%) | 2 (22.2%) | 11 (55%) | 12 (70.6%) | 7 (70%) |
| CFRD | 11 (19.6 %) | 0 (0%) | 3 (15%) | 6 (35.3%) | 2 (20%) |
| CT score | 36.5 (18.25, 57.5) | 8 (7, 22) | 30.5 (18.5, 50) | 44 (33.5, 64) | 61.5 (17, 72) |
| FEV1% | 74.3 ± 21.7 | 86.33 ± 11.5 | 75.4 ± 22.4 | 74.35 ± 23.1 | 61.2 ± 20.7 |
| FEF25–75% | 62.84 ± 27.5 | 75.56 ± 15.2 | 63.10 ± 26.4 | 65.82 ± 30.4 | 45.8 ± 28.4 |
| Sex | |||||
| female | 26 (46.4%) | 4 (44.4%) | 9 (45%) | 8 (47.1%) | 5 (50%) |
| male | 30 (53.6%) | 5 (55.6%) | 11 (55%) | 9 (52.9%) | 5 (50%) |
| Genotype F508del | |||||
| homozygous heterozygous non-F508del mutation | 26 (46.4%) 26 (46.4%) 4 (7.2%) | 0 (%) 8 (88.9%) 1 (11.1%) | 9 (45%) 9 (45%) 2 (10%) | 11 (64.7%) 5 (29.4%) 1 (5.9%) | 6 (60%) 4 (40%) 0 (0%) |
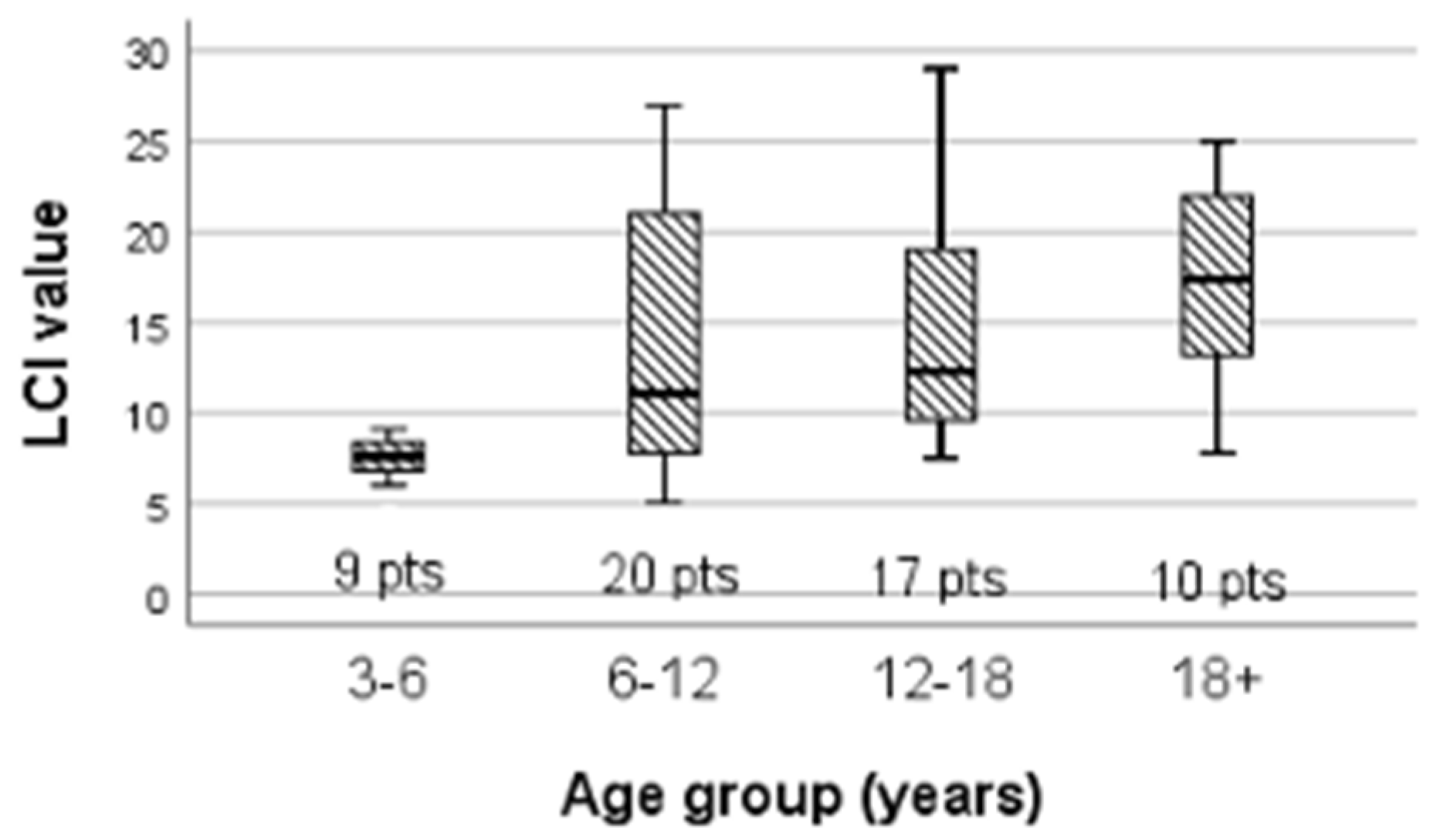
| LCI | 11.58 (7.88, 19.83) | 7.6 (6.4, 8.765) | 11.1 (7.78, 21.05) | 12.3 (9.135, 22) | 17.38 (13.2, 22.00) |
| Pse. chronic infection | 25 (44.6%) | 1 (11.1%) | 5 (25%) | 10 (58.8%) | 9 (90%) |
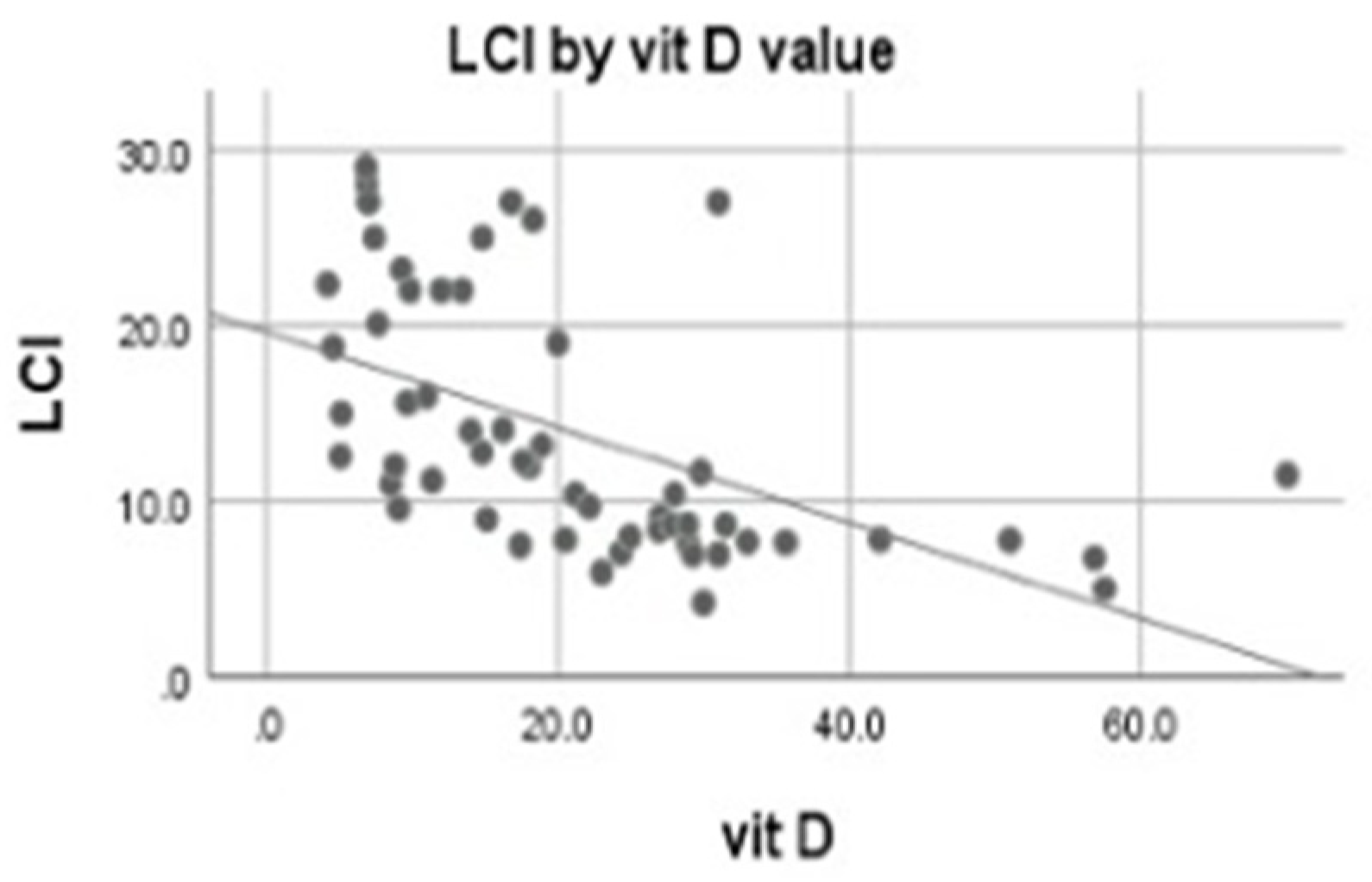
| Vitamin D ng/mL | 18.13 (9.68, 28.93) | 27 (24.635, 43.39) | 17.53 (11.7, 30.4) | 17.4 (7.15, 24.6) | 12.21 (9.63, 18.9) |
| Median Vit D | Mean Rank Vit D | Mann-Whitney U | p Value | |
|---|---|---|---|---|
| BMI percentile <25th vs. >25th | 13.4 vs. 28.9 | 22.1 vs. 37.6 | 169.5 | 0.000 |
| Age group >18 years vs. <18 years | 12.2 vs. 20.3 | 20.5 vs. 30.2 | 150 | 0.087 |
| F508del mutation homozygote vs. non-homozygote | 15.5 vs. 23.7 | 24.4 vs. 32 | 284.5 | 0.083 |
| RR | 95% CI | ||
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| CFLD | 2.87 | 0.83 | 9.94 |
| CFRD | 1.92 | 0.28 | 13.17 |
| Pse. chronic infection | 1.00 | 0.45 | 2.23 |
| FEV1 < 80% | 4.97 | 0.77 | 31.16 |
| FEF25–75 < 80% | 1.34 | 0.73 | 2.46 |
| Median LCI | Mean Rank LCI | Mann-Whitney U | p Value | |
|---|---|---|---|---|
| BMI percentile | ||||
| <25th vs. >25th | 14 vs. 8 | 34.5 vs. 19.9 | 182.5 | 0.001 |
| CFLD yes vs. no | 14 vs. 8.7 | 34.4 vs. 20.6 | 194.5 | 0.002 |
| CFRD yes vs. no | 12.6 vs. 11 | 35 vs. 26.9 | 176.0 | 0.140 |
| FEV1 < 80% yes vs. no | 15.6 vs. 8.6 | 36.2 vs. 21.3 | 183.0 | 0.001 |
| FEF25–75 < 80% | ||||
| yes vs. no | 13.6 vs. 7.8 | 33.3 vs. 16.5 | 128.0 | 0.000 |
| Genotype F508del | ||||
| homozygote vs non-del homozygote | 12.6 vs. 9.4 | 33.6 vs. 24.1 | 258.0 | 0.030 |
| Pse. chronic infection | ||||
| yes vs. no | 18.8 vs. 9.1 | 36.6 vs. 21.9 | 184.0 | 0.001 |
| Vitamin D status | ||||
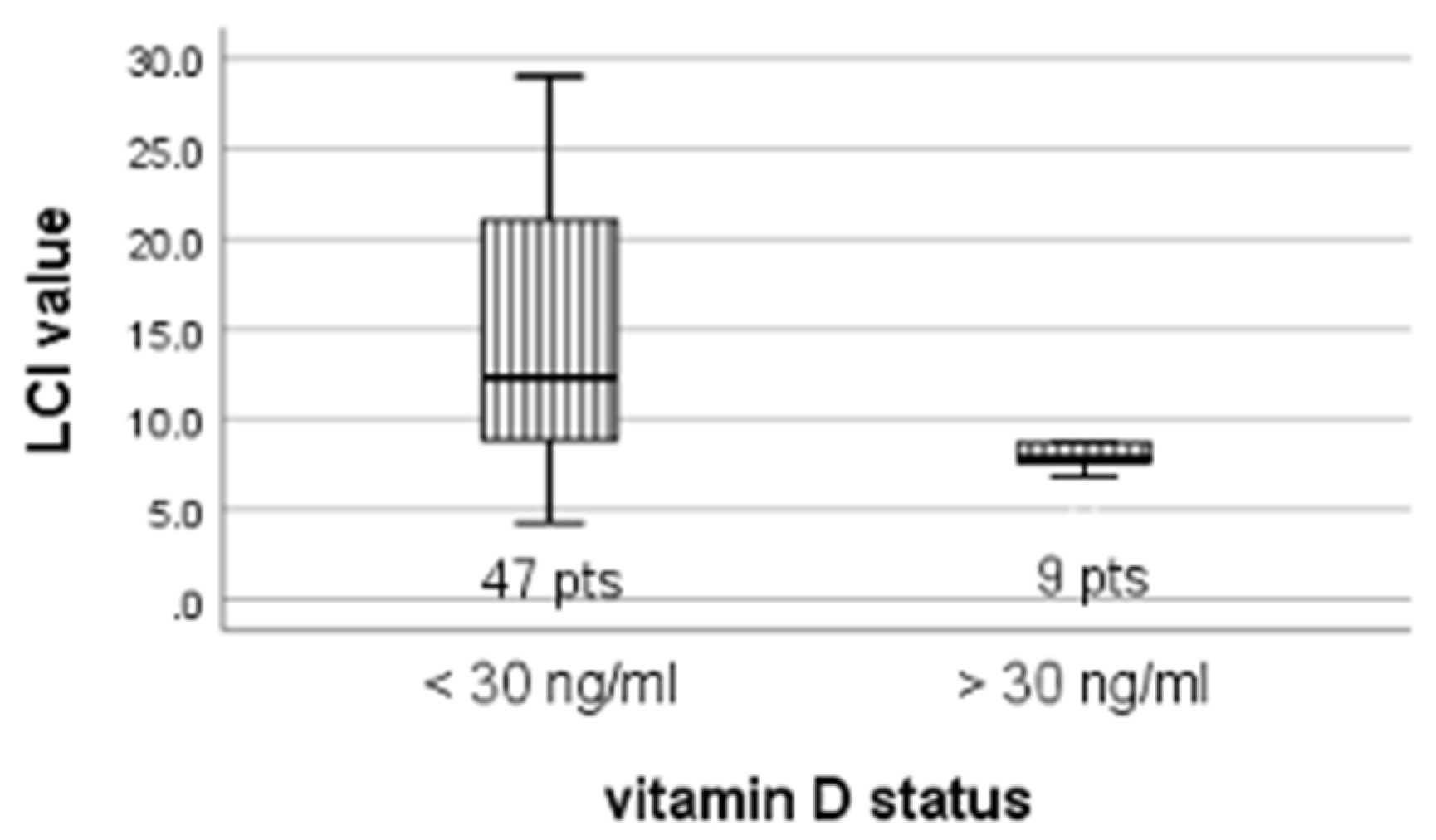
| <30 ng/mL vs. >30 ng/mL | 12.3 vs. 7.8 | 20.7 vs. 16.8 | 106.5 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dediu, M.; Ciuca, I.M.; Pop, L.L.; Iacob, D. The Relation between Vitamin D Level and Lung Clearance Index in Cystic Fibrosis—A Pilot Study. Children 2022, 9, 329. https://doi.org/10.3390/children9030329
Dediu M, Ciuca IM, Pop LL, Iacob D. The Relation between Vitamin D Level and Lung Clearance Index in Cystic Fibrosis—A Pilot Study. Children. 2022; 9(3):329. https://doi.org/10.3390/children9030329
Chicago/Turabian StyleDediu, Mihaela, Ioana Mihaiela Ciuca, Liviu Laurentiu Pop, and Daniela Iacob. 2022. "The Relation between Vitamin D Level and Lung Clearance Index in Cystic Fibrosis—A Pilot Study" Children 9, no. 3: 329. https://doi.org/10.3390/children9030329
APA StyleDediu, M., Ciuca, I. M., Pop, L. L., & Iacob, D. (2022). The Relation between Vitamin D Level and Lung Clearance Index in Cystic Fibrosis—A Pilot Study. Children, 9(3), 329. https://doi.org/10.3390/children9030329







