Supracondylar Fractures of the Humerus: Association of Neurovascular Lesions with Degree of Fracture Displacement in Children—A Retrospective Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Demographic and Baseline Assessments
2.2. Clinical and Radiographic Assessments
2.3. Treatments
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
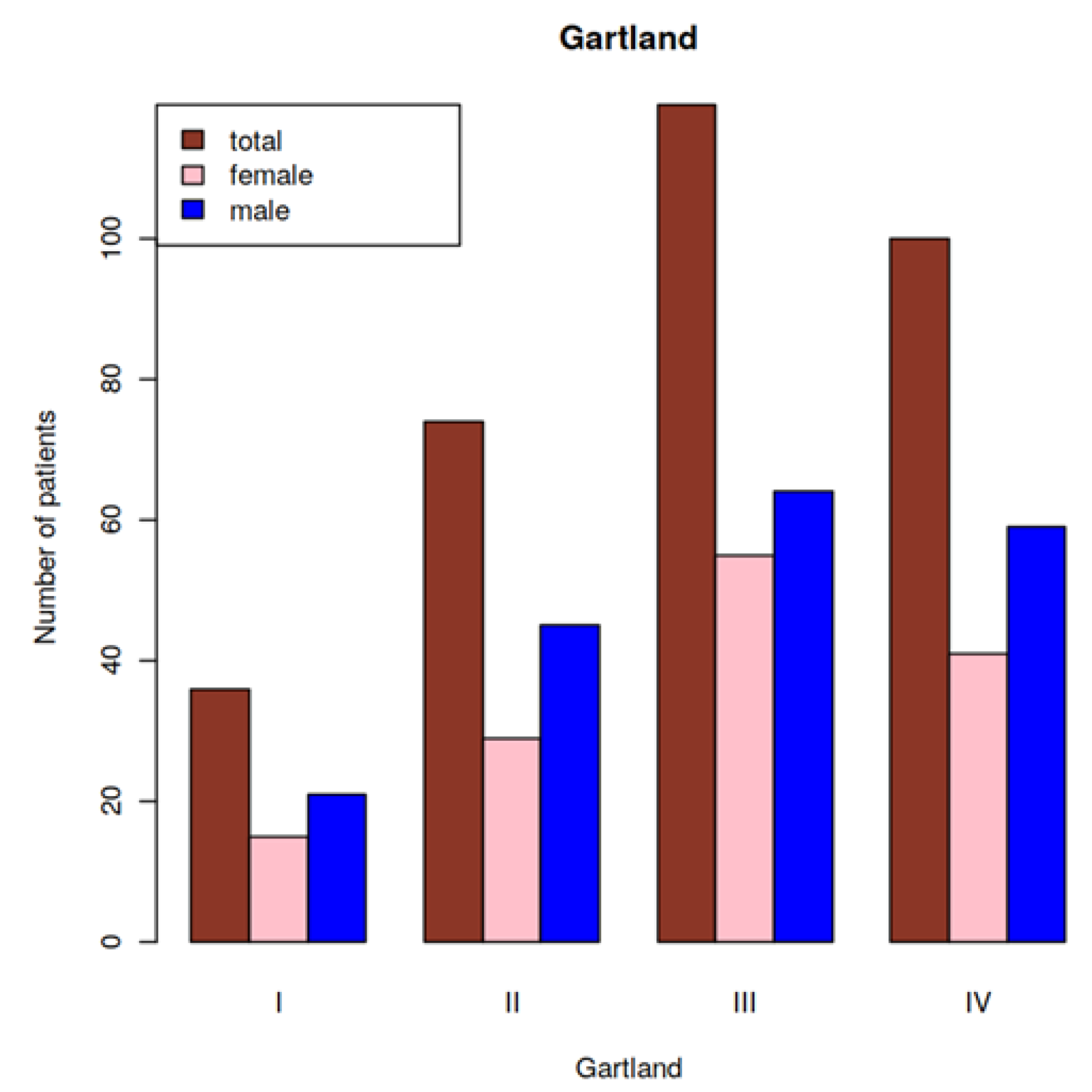
3.1. Patient Demographics and Number of Patients per Gartland Type at Baseline
3.2. Relationship between the Incidence of Neurovascular Lesions and the Gartland Score
Types of Nerve Injuries Associated with ScHF
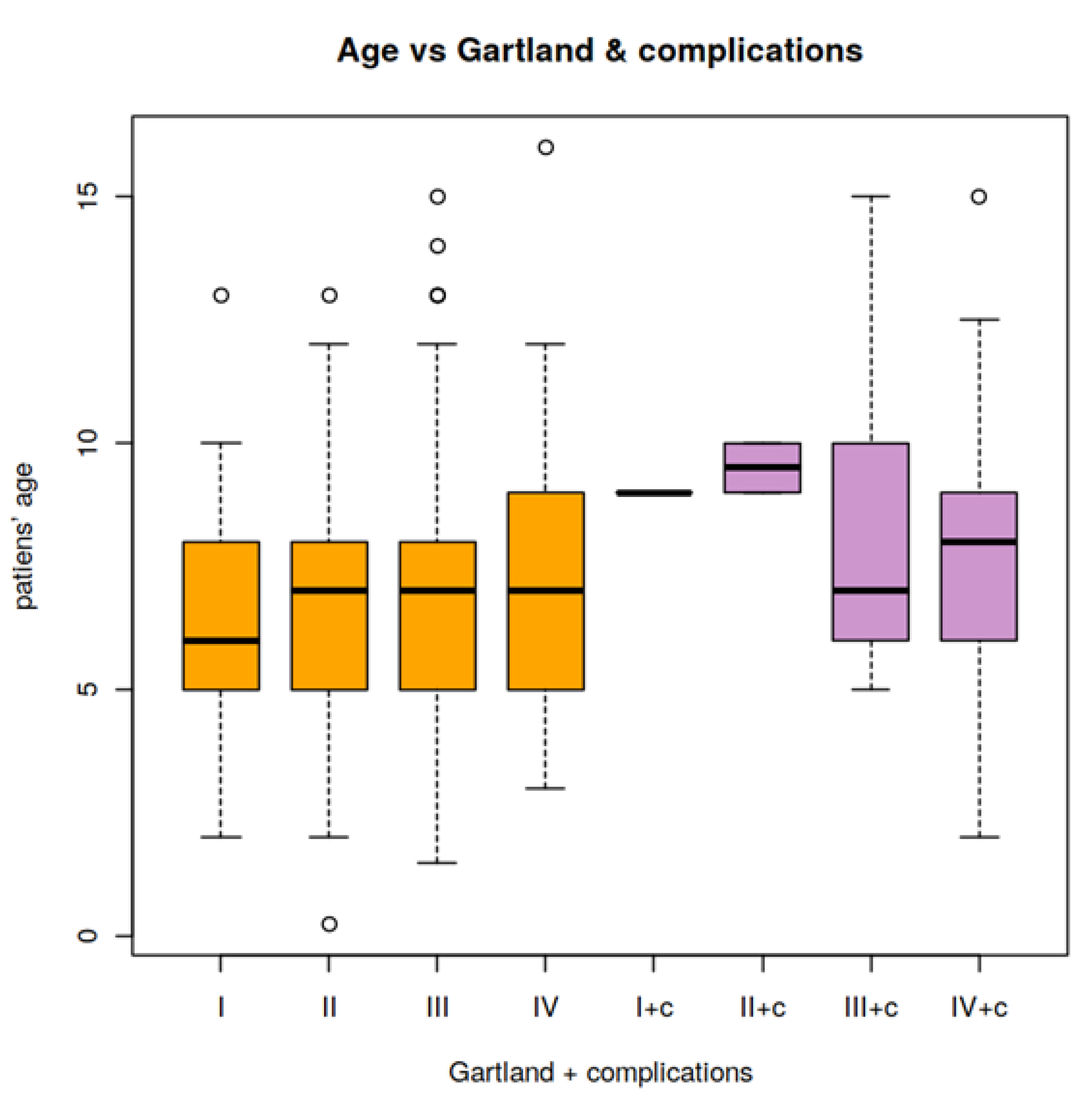
3.3. Relationship of the Patient Age and the Presence of Neurovascular Lesions
3.4. Relationship of Neurovascular Lesions, Gartland Type, and Patient Gender
3.5. Relationship of Fracture Laterality (Right or Left) on the Gartland Score
3.6. Outcome of Nerve Lesions and Vascular Injuries
4. Discussion
4.1. Incidence of Nerve Lesions
4.1.1. Types of Nerve Injuries Associated with ScHF
4.1.2. Assessment of Neurologic Function
4.2. Iatrogenic Nerve Injuries
4.3. Incidence of Non-Iatrogenic Vascular Injuries
4.4. Combined Vascular and Neurologic Lesions
4.5. Study Limitations and Strengths
4.5.1. Study Limitations
4.5.2. Study Strengths
5. Conclusions
- The incidence of neurovascular complications was related to the degree of ScHF displacement as classified according to Gartland. Vascular complications mainly accompanied Gartland type IV ScHF, whereas nerve lesions occurred in Gartland type III and IV ScHF.
- For the treatment of displaced ScHF, we recommend closed reduction and stabilization by K-wires inserted percutaneously from the lateral aspect of the distal humerus. If the impaired perfusion of the forearm persists after fracture reduction and stabilization or if complete nerve paralysis or iatrogenic nerve lesion develops, surgical treatments of these neurovascular complications should be considered.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ScHF | supracondylar humerus fracture |
| AP | anteroposterior |
References
- Houshian, S.; Mehdi, B.; Larsen, M.S. The epidemiology of elbow fracture in children: Analysis of 355fractures, with special reference to supracondylar humerus fractures. J. Orthop. Sci. 2001, 6, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Omid, R.; Choi, P.D.; Skaggs, D.L. Supracondylar humeral fractures in children. J. Bone Jt. Surg. 2008, 90, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.Y.; Lam, T.P.; Shen, W.Y. Closed Reduction and Percutaneous Pinning for Type III Displaced Supracondylar Fractures of the Humerus in Children. J. Orthop. Trauma 1995, 9, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Lam, T.P.; Maffulli, N. Epidemiological features of supracondylar fractures of the humerus in Chinese children. J. Pediatr. Orthop. 2001, 10, 63–67. [Google Scholar]
- Mazzini, J.P.; Rodriguez-Martin, J.; Andres-Esteban, E.M. Does open reduction and pinning affect outcome in severely displaced supracondylar humeral fractures in children? A systematic review. Strat. Trauma Limb Reconstr. 2010, 5, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Farnsworth, C.L.; Silva, P.D.; Mubarak, S.J. Etiology of Supracondylar Humerus Fractures. J. Pediatr. Orthop. 1998, 18, 38–42. [Google Scholar] [CrossRef]
- Gartland, J.J. Management of supracondylar fractures of the humerus in children. Surg. Gynecol. Obstet. 1959, 109, 145–154. [Google Scholar]
- Rockwood, C.A.; Beaty, J.H.; Kasser, J.R. Rockwood and Wilkins’ Fractures in Children; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Agus, H.; Kalenderer, Ö.; Kayali, C. Closed reduction and percutaneous pinning results in children with supracondylar humerus fractures. Acta Orthop. Traumatol. Turc. 1999, 33, 18–22. [Google Scholar]
- Pavone, V.; Riccioli, M.; Testa, G.; Lucenti, L.; De Cristo, C.; Condorelli, G.; Avondo, S.; Sessa, G. Surgical Treatment of Displaced Supracondylar Pediatric Humerus Fractures: Comparison of Two Pinning Techniques. J. Funct. Morphol. Kinesiol. 2016, 1, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Terpstra, S.E.S.; Burgers, P.T.B.W.; Huub, J.L.; van der Heide, H.J.L.; Bas de Witte, P. Pediatric Supracondylar Humerus Fractures: Should We Avoid Surgery during After-Hours? Children 2022, 9, 189. [Google Scholar] [CrossRef]
- Cramer, K.E.; Green, N.E.; Devito, D.P. Incidence of Anterior Interosseous Nerve Palsy in Supracondylar Humerus Fractures in Children. J. Pediatr. Orthop. 1993, 13, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Joiner, E.R.; Skaggs, D.L.; Arkader, A.; Andras, L.M.; Lightdale-Miric, N.R.; Pace, J.L.; Ryan, D.D. Iatrogenic nerve injuries in the treatment of supracondylar humerus fractures: Are we really just missing nerve injuries on preoperative examination? J. Pediatr. Orthop. 2014, 34, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.; Moraleda, L.; Díez-Sebastián, J. Long-term Functional Results of Neurological Complications of Pediatric Humeral Supracondylar Fractures. J. Pediatr. Orthop. 2015, 35, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.C.; Zinar, D.M. Traumatic and Iatrogenic Neurological Complications After Supracondylar Humerus Fractures in Children. J. Pediatr. Orthop. 1995, 15, 440–443. [Google Scholar] [CrossRef]
- McGraw, J.J.; Akbarnia, B.A.; Hanel, D.P.; Keppler, L.; Burdge, R.E. Neurological Complications Resulting from Supracondylar Fractures of the Humerus in Children. J. Pediatr. Orthop. 1986, 6, 647–650. [Google Scholar] [CrossRef]
- Brahmamdam, P.; Plummer, M.; Modrall, J.G.; Megison, S.M.; Clagett, G.P.; Valentine, R.J. Hand ischemia associated with elbow trauma in children. J. Vasc. Surg. 2011, 54, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabharwal, S.; Tredwell, S.J.; Beauchamp, R.D.; Mackenzie, W.G.; Jakubec, D.M.; Cairns, R.; LeBlanc, J.G. Management of Pulseless Pink Hand in Pediatric Supracondylar Fractures of Humerus. J. Pediatr. Orthop. 1997, 17, 303–310. [Google Scholar] [CrossRef]
- Noaman, H.H. Microsurgical reconstruction of brachial artery injuries in displaced supracondylar fracture humerus in children. Microsurgery 2006, 26, 498–505. [Google Scholar] [CrossRef]
- Wegmann, H.; Eberl, R.; Kraus, T.; Till, H.; Eder, C.; Singer, G. The impact of arterial vessel injuries associated with pediatric supracondylar humeral fractures. J. Trauma Acute Care Surg. 2014, 77, 381–385. [Google Scholar] [CrossRef]
- Wang, J.H.; Morris, W.Z.; Bafus, B.T.; Liu, R.W. Pediatric Supracondylar Humerus Fractures: AAOS Appropriate Use Criteria Versus Actual Management at a Pediatric Level 1 Trauma Center. J. Pediatr. Orthop. 2019, 39, e578–e585. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.G.; Robertson, J.S.; Godman, A.; Boyle, J.; Huntley, J.S. Introduction of a simple guideline to improve neurological assessment in paediatric patients presenting with upper limb fractures. Emerg. Med. J. 2016, 33, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Vallila, N.; Sommarhem, A.; Paavola, M.; Nietosvaara, Y. Pediatric distal humeral fractures and complications of treatment in Finland: A review of compensation claims from 1990 through 2010. J. Bone Jt. Surg. Am. 2015, 97, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Islam, A.; Puri, R. Current Management of Paediatric Supracondylar Fractures of the Humerus. Cureus 2020, 12, e8137. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Riccioli, M.; Culmone, A.; Cosentino, P.; Caponnetto, M.; Dimartino, S.; Testa, G. Is Supine Position Superior to Prone Position in the Surgical Pinning of Supracondylar Humerus Fracture in Children? J. Funct. Morphol. Kinesiol. 2020, 5, 57. [Google Scholar] [CrossRef]
- Schmid, T.G.J.; Joeris, A.; Slongo, T.; Ahmad, S.S.; Ziebarth, K. Displaced supracondylar humeral fractures: Influence of delay of surgery on the incidence of open reduction, complications and outcome. Arch. Orthop. Trauma Surg. 2015, 135, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Leversedge, F.J.; Moore, T.J.; Peterson, B.C.; Seiler, J.G., III. Compartment syndrome of the upper extremity. J. Hand Surg. 2011, 36, 544–560. [Google Scholar] [CrossRef]
- Babal, J.C.; Mehlman, C.T.; Klein, G. Nerve Injuries Associated with Pediatric Supracondylar Humeral Fractures: A Meta-analysis. J. Pediatr. Orthop. 2010, 30, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Ristic, S.; Strauch, R.J.; Rosenwasser, M.P. The Assessment and Treatment of Nerve Dysfunction After Trauma Around the Elbow. Clin. Orthop. Relat. Res. 2000, 370, 138–153. [Google Scholar] [CrossRef]
- Culp, R.W.; Osterman, A.L.; Davidson, R.S.; Skirven, T.; Bora, F.W. Neural injuries associated with supracondylar fractures of the humerus in children. J. Bone Jt. Surg. 1990, 72, 1211–1215. [Google Scholar] [CrossRef]
- Harris, N.; Ali, F. (Eds.) Examination Techniques in Orthopaedics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Barrett, K.K.; Skaggs, D.L.; Sawyer, J.R.; Andras, L.; Moisan, A.; Goodbody, C.; Flynn, J.M. Supracondylar Humeral Fractures with Isolated Anterior Interosseous Nerve Injuries: Is Urgent Treatment Necessary? J. Bone Jt. Surg. 2014, 96, 1793–1797. [Google Scholar] [CrossRef]
- Begovic, N.; Paunovic, Z.; Djuraskovic, Z.; Lazovic, L.; Mijovic, T.; Babic, S. Lateral pinning versus others procedures in the treatment of supracondylar humerus fractures in children. Acta Orthop. Belg. 2016, 82, 866–871. [Google Scholar] [PubMed]
- Pesenti, S.; Ecalle, A.; Gaubert, L.; Peltier, E.; Choufani, E.; Viehweger, E.; Jouve, J.-L.; Launay, F. Operative management of supracondylar humeral fractures in children: Comparison of five fixation methods. Orthop. Traumatol. Surg. Res. 2017, 103, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.; Firoozbakhsh, K.; Passarelli, R.; Bosch, P. Biomechanical Analysis of Pinning Techniques for Pediatric Supracondylar Humerus Fractures. J. Pediatr. Orthop. 2006, 26, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Afaque, S.F.; Singh, A.; Maharjan, R.; Ranjan, R.; Panda, A.K.; Mishra, A. Comparison of clinic-radiological outcome of cross pinning versus lateral pinning for displaced supracondylar fracture of humerus in children: A randomized controlled trial. J. Clin. Orthop. Trauma 2020, 11, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, D.S.; Leitch, K.; Wright, J.G. The treatment of supracondylar fractures in children with an absent radial pulse. J. Pediatr. Orthop. 1996, 16, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Louahem, D.M.; Nebunescu, A.; Canavese, F.; Dimeglio, A. Neurovascular complications and severe displacement in supracondylar humerus fractures in children: Defensive or offensive strategy? J. Pediatr. Orthop. B 2006, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]

| Gender (female/male; %) | 140 (42.6%)/189 (57.4%) |
| Age (years; CI) | 7.2 (CI: 6.89, 7.45) |
| Ethnicity (n; %) | Caucasian (326; 99.1%); Asian (2; 0.61%); African (1; 0.3%) |
| All Patients (n = 329) | Mean and CI | Variation and CI |
|---|---|---|
| Gartland score | 2.86 CI = 2.75–2.97 | 0.95 CI = 0.82–1.11 |
| Age (years) | 7.2 CI = 6.89–7.45 | 6.76 CI = 5.84–7.93 |
| Number of Patients | Gartland Type | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Total (n = 329) | 36 | 74 | 119 | 100 |
| With neurovascular lesions (group 1; n = 44) | 1 | 2 | 14 | 27 |
| Without neurovascular lesions (group 2; n = 285) | 35 | 72 | 105 | 73 |
| Patients with Neurovascular Lesions (Group 1, n = 44) | Patients without Neurovascular Lesions (Group 2, n = 285) | p-Value | |||
|---|---|---|---|---|---|
| Mean and CI | Variation and CI | Mean and CI | Variation and CI | ||
| Gartland score | 3.52 CI = 3.31–3.73 | 0.48 CI = 0.33–0.78 | 2.75 CI = 2.64–2.87 | 0.94 CI = 0.81–1.12 | 0.045 |
| Age (years) | 8.2 CI = 7.32–9.02 | 7.81 CI = 5.33–12.54 | 7.0 CI = 6.72–7.31 | 6.45 CI = 5.51–7.66 | 0.04 |
| Number of Patients with Neurovascular Lesions | Gartland Type | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Total (n = 44) | 1 | 2 | 14 | 27 |
| Nerve lesions (n = 24) | 1 | 2 | 11 | 10 |
| Vascular lesions (n = 12) | 0 | 0 | 1 | 11 |
| Combined neurovascular injuries (n = 8) | 0 | 0 | 2 | 6 |
| Type of Predominant Nerve Lesion | Gartland Type | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Median nerve lesion | 1 | 2 | 6 | 9 |
| Anterior interosseous nerve lesion | 0 | 0 | 3 | 4 |
| Ulnar nerve lesion | 0 | 0 | 3 | 3 |
| Radial nerve lesion | 0 | 0 | 1 | 0 |
| All Patients (n = 329) | Gartland Type | |||
|---|---|---|---|---|
| I | II | III | IV | |
| Mean age and CI (years) | 6.4 CI = 5.63–7.26 | 6.9 CI = 6.39–7.5 | 7.4 CI = 6.87–7.85 | 7.4 CI = 6.86–7.91 |
| Variation | 5.74 CI = 3.78–9.77 | 5.73 CI = 4.25–8.16 | 7.31 CI = 5.76–9.61 | 7.12 CI = 5.49–9.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewski, R.; Pethe, K.; Kler, J.; Rutz, E.; Mayr, J.; Dajka, J. Supracondylar Fractures of the Humerus: Association of Neurovascular Lesions with Degree of Fracture Displacement in Children—A Retrospective Study. Children 2022, 9, 308. https://doi.org/10.3390/children9030308
Tomaszewski R, Pethe K, Kler J, Rutz E, Mayr J, Dajka J. Supracondylar Fractures of the Humerus: Association of Neurovascular Lesions with Degree of Fracture Displacement in Children—A Retrospective Study. Children. 2022; 9(3):308. https://doi.org/10.3390/children9030308
Chicago/Turabian StyleTomaszewski, Ryszard, Karol Pethe, Jacek Kler, Erich Rutz, Johannes Mayr, and Jerzy Dajka. 2022. "Supracondylar Fractures of the Humerus: Association of Neurovascular Lesions with Degree of Fracture Displacement in Children—A Retrospective Study" Children 9, no. 3: 308. https://doi.org/10.3390/children9030308
APA StyleTomaszewski, R., Pethe, K., Kler, J., Rutz, E., Mayr, J., & Dajka, J. (2022). Supracondylar Fractures of the Humerus: Association of Neurovascular Lesions with Degree of Fracture Displacement in Children—A Retrospective Study. Children, 9(3), 308. https://doi.org/10.3390/children9030308








