Abstract
Background: Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor with intermediate malignancy that tends to affect children primarily. To date, no standardized therapies exist for the treatment of IMT. This study aimed to share experience from China Children’s Medical Center for the explorative treatment of IMT. Methods: Patients with newly diagnosed IMT between January 2013 and December 2018 were included. Patients were grouped according to surgical margins and Intergroup Rhabdomyosarcoma Study Group (IRSG) staging. The clinical characteristic, therapeutic schedules, treatment response and clinical outcome were described. Results: Six patients were enrolled in this study, including two boys and four girls, with a median age of 57 months (range 10–148 months). Among them, five patients were anaplastic lymphoma kinase positive. Four patients achieved complete remission and two patients attained partial remission after treatment with this protocol. All patients were alive after a median follow-up of 4 years (range 3–7 years). The most common treatment-related adverse reaction was myelosuppression. Conclusion: In this study, we demonstrated that IMT has a good prognosis and the treatment selected according to risk stratification was effective and feasible.
1. Introduction
An inflammatory myofibroblastic tumor (IMT), originally known as an inflammatory pseudotumor, has the potential of recurrence and aggressive behavior [1]. The malignancy is also called by some other terms such as pseudosarcomatous myofibroblastic proliferation or inflammatory myofibrohistiocytic proliferation, which involves the myofibroblastic spindle cells and the infiltration of inflammatory lymphoplasma cells and eosinophils. In addition to its common occurrence in the lungs, IMT can also occur in various extrapulmonary sites including colon, genital tract, spleen, and orbital region [1,2]. About 30% of the patients with IMT may have clinical manifestations of inflammation symptoms.
IMT is defined as an intermediate soft tissue tumor by the World Health Organization, as it can be locally invasive and reoccur [3]. Multifocal disease and distant metastases are infrequent in IMT. Nevertheless, epithelioid inflammatory myofibroblastic sarcoma (EIMS), a unique subtype of IMT, has a relatively high incidence of local recurrence and distant metastases [4]. Surgery remains the mainstay of treatment for localized IMT, whereas systemic therapy is applicable in advanced disease or inoperable tumor sites. Conventional therapies include high-dose corticosteroids, non-steroidal anti-inflammatory drugs, cyclosporine A, vinblastine/methotrexate or doxorubicin- based chemotherapy [5,6], and local radiotherapy. As activating rearrangements of the anaplastic lymphoma kinase (ALK) gene were identified in 50% of cases, the targeted inhibition of ALK is a promising treatment option for IMT [7]. Other actionable genomic alterations, including ROS1, RET, NTRK, and PDGFRβ infusion, are also potential therapeutic targets [8]. Although various approaches have witnessed huge progress in the treatment of IMT, the development of standardized systemic therapy and effective clinical management approaches is urgently required. China Children’s Medical Center (CCMC) was the first to perform risk stratification for IMT. We demonstrated treatment diversity depending on risk grouping in this retrospective study.
2. Patients and Methods
2.1. Patient
Patients aged less than 18 years, newly diagnosed with IMT and previously untreated, were selected for the study from January 2013 to December 2018. The diagnosis of IMT was based on clinical presentation, radiology, conventional histology and immunohistology, and molecular pathology. The ALK status was determined by immunohistochemical evaluations. The clinical data were collected from the medical records. This study was approved by the Ethics and Scientific Committee of Hubei University of Medicine with approval number XH2021004. The patients’ parents gave written informed consent before the therapy was given.
2.2. Treatment
Table 1 lists the detailed group criteria and treatment regimens of our study. Patients in Group I received surgery only at the time of diagnosis. Patients classified into Groups II and Group III received two to four cycles of vincristine, doxorubicin, and cyclophosphamide (VDC) regimen, followed by surgery. Intensive low-dose vincristine plus methotrexate (MTX-V) regimen was adopted for patients in Group IV. The MTX-V regimen was also selected for patients in Group III with localized lesions at an inoperable location. Local radiotherapy was used alternatively in patients with distant metastases or macroscopic-positive resection. Topotecan plus cyclophosphamide (TC) regimen was selected as the second-line chemotherapy for relapsed patients. Moreover, when a patient presented with a positive ALK status, crizotinib was also recommended and orally administered every day for 1 year.

Table 1.
Clinical group and treatment in China Children’s Medical Center (CCMC).
3. Treatment Response and Toxicity
A modified Response Evaluation Criteria in Solid Tumors (RECIST) was used to assess treatment response [9]. Complete remission (CR) was defined as the absence of all lesions for more than 4 weeks. Partial remission (PR) was characterized as a more than 64% decrease in the size of primary tumor and more than 30% reduction in metastatic lesions, with no new metastatic lesions. Progressive disease (PD) was defined as a more than 40% increase in the size of primary tumor or the appearance of new lesions. Stable disease was between PR and PD.
The necessary examination, including hematological and biochemistry tests, electrocardiogram, and electrocardiograph, was completed before every cycle of chemotherapy. Treatment-related adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [10].
4. Results
4.1. Patients
From January 2013 to December 2018, six patients newly diagnosed with IMT were enrolled in our study, including two boys and four girls. The median onset age was 57 months (range 10–148). Five patients demonstrated the activation of ALK. One patient had the EIMS subtype. The clinical and biological characteristics of all cases are shown in Table 2.

Table 2.
Clinical characteristics, treatment-related toxicities and treatment response of 6 patients with inflammatory myofibroblastic tumor (IMT).
4.2. Treatment and Toxicity
The chemotherapy regimens used in this study are shown in Table 3. Patients in Group I underwent initial complete resection without any postoperative adjuvant treatment. Patients in Groups II-III underwent R1/R2 surgery, then received 2–4 cycles of adjuvant chemotherapy. Systemic chemotherapy was the main treatment for patients in Group IV. Radiotherapy could be considered for patients with respiratory distress syndrome, who did not show rapid response to chemotherapy or who could not tolerate chemotherapy. Only one patient received radiation after incomplete surgical resection at the prescribed dose of 24 Gy (5 × 1.8 Gy fractions per week).

Table 3.
The vincristine, doxorubicin, and cyclophosphamide (VDC) regimen, vincristine plus methotrexate (MTX-V) regimen and topotecan plus cyclophosphamide (TC) regimen.
The common adverse reactions were myelosuppression and gastrointestinal reaction. Three patients experienced febrile neutropenia. Four patients suffered from vomiting and diarrhea. One patient in Group IV developed numbness due to the long-term use of vinorelbine. The discomfort was alleviated by symptomatic treatment. Overall, the adverse reactions were tolerable and manageable.
4.3. Treatment Response and Clinical Outcome
All of the patients were alive during a follow-up period of at least 3 years. Patients in Group I and Patients in Group II-III who received adjuvant chemotherapy achieved CR with no reports of recurrence. One patient in Group II with ALK positive received radiotherapy after surgery, without adjuvant chemotherapy, and experienced systemic relapses twice. She achieved the first remission after treatment with MTX-V regimen combined with crizotinib. A second prolonged remission was achieved when the patient was treated with second-line chemotherapy (topotecan plus cyclophosphamide) combined with crizotinib. Of the two patients in Group IV, one patient achieved CR and another patient achieved PR.
5. Discussion
Although IMT was first described in 1937, its etiology remains unclear or unknown. ALK was recognized as an oncogenic driver in lymphoma and some solid tumors [11,12]. Since all patients with EIMS and 50% of the patients with IMT patients present with the activation of ALK, there is no doubt that IMT is a true neoplasm rather than a reaction to inflammation. Therefore, active treatment is necessary.
The dominance of surgery has been well-established in resectable lesions. The question was whether postoperative chemotherapy was required. Several studies showed that a small proportion of patients experienced local recurrence within 3 months after surgical resection without adjuvant therapy [7], which might provide evidence for the need for adjuvant postoperative chemotherapy. According to the experience from the European pediatric Soft Tissue Sarcoma Study Group (EpSSG), the “wait and watch” strategy could be adopted for patients in Group I and systemic chemotherapy could be administered to patients in Group III; whether adjuvant chemotherapy was necessary for patients in Group II was still controversial [13]. Which chemotherapy had a high degree of activity in IMT was another question that needed to be addressed. One European retrospective study demonstrated that anthracycline-based regimens remained the front-line standard treatment of IMT [6]. However, a multicenter retrospective case-series analysis indicated that the MTX-V regimen had a similar object remission rate (ORR) compared with the anthracycline-based regimens in advanced IMT (54% versus 48%); more importantly, the MTX-V regimen had more prolonged disease control [14]. In our study, patients in Group II and III were treated with VDC regimen and MTX-V regimen was given to patients in Group IV and those with challenging primary sites that were inoperable. This indicated that some pediatric patients might have been overtreated in this retrospective study. Considering the cardiotoxicity of anthracycline and the risk of secondary tumor caused by alkylating agents, we are more likely to recommend the MTX-V regimen as the first-line treatment for advanced IMT in the future.
Whether patients with a positive resection margin can be benefit from radiation is still uncertain. In our study, patient 3 (the age at diagnosis was 123 months) with R1 section in the primary tumor site was given local external irradiation without chemotherapy. She did not experience local recurrence after the 4-year follow-up, but new lesions were found in other sites. Considering the long-term sequelae such as growth retardation caused by radiation in very young children, we held the opinion that radiotherapy was not recommended for patients aged less than 3 years, no matter which group they were classified in. For patients aged more than 3 years in Group IV, radiation could be used as an alternative therapy because IMT had a good prognosis, but it must be individualized.
ALK inhibition proved to be highly effective and was recommended as a first-line treatment for IMT considered ALK positive by the Children’s Oncology Group [15,16]. However, ALK inhibitors may be unavailable for patients in developing countries because of the high cost. Moreover, many questions concerning the treatment duration, the mechanism of drug resistance, and the management of drug resistance remain unanswered. Therefore, crizotinib was administered as a second-line treatment for the only refractory patient in our study. A study showed that the acquired drug resistance to crizotinib resulting from the ALK (G1269A) mutation was reversible by the next-generation ALK inhibitor [17,18]. Rituximab might be beneficial for ALK-negative recurrent IMT [19].
We could not recruit enough patients in a single institute because of the rarity of IMT and the young age at onset; therefore, it was difficult for us to evaluate the effectiveness of this protocol based on this risk stratification, and we did not analyze the long-term survival. So far, no consistent guideline recommendation exists for clinicians because most studies on IMT are sporadic case reports. We formulated a treatment strategy for IMT based on the experience from EpSSG and CCMC to help the clinicians (Figure 1). However, we realized that further research with both a larger sample and multiple centers is still needed.
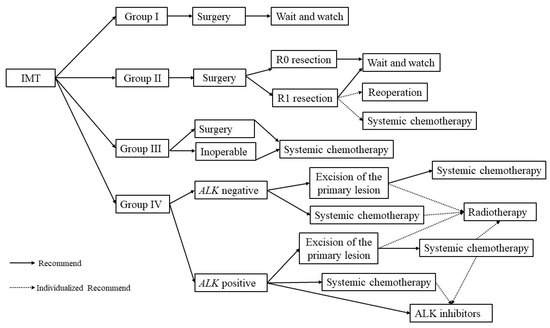
Figure 1.
The treatment strategy for IMT.
In conclusion, we formulated the treatment strategy for IMT according to risk stratification. Surgery was still the mainstream treatment. A “wait and watch” strategy was applied for patients in Group I. Whether adjuvant treatment was needed after surgery for patients in Group II should be individualized. Systemic chemotherapy should be given to patients in Group III after surgical resection or biopsy alone. Systemic chemotherapy was the main treatment for patients in Group IV, ALK inhibitors might be an alternative choice for patients who are ALK positive. Even so, further studies with a larger patient population are needed to evaluate the treatment efficiency.
Author Contributions
Conceptualization, D.Z. and P.H.; data curation and writing, writing—review and editing, Y.D., Y.H. and K.R.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Innovative Research Program of Xiangyang No. 1 People’s Hospital (Grants number: XYY2021Q02) and Key projects of Xiangyang Science and Technology Bureau (2021YL26).
Institutional Review Board Statement
This study was approved by the Ethics and Scientific Committee of Hubei University of Medicine and was performed according to the Good Clinical Practice Guidelines and the Helsinki Declaration. All the patients gave written informed consent before enrollment.
Informed Consent Statement
This manuscript could be sent to publication when it was accepted; consent to publish had been obtained from the children’s parents.
Data Availability Statement
The clinical data supporting the conclusions of this manuscript will be made available by the authors.
Acknowledgments
We thank the patients and clinicians who participated in this study and the support from Shanghai Children Medical Center and Xinhua hospital. We thank Kaweme Natasha for the help to revise our manuscript.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. The authors declare that they have no competing interest.
References
- Krings, G.; McIntire, P.; Shin, S.J. Myofibroblastic, fibroblastic and myoid lesions of the breast. Semin. Diagn. Pathol. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, B.; Roy, P.S.; Gupta, K.; Sekar, A.; Bansal, D. Infantile Inflammatory Myofibroblastic Tumor of Spleen. Fetal Pediatr. Pathol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.J.; Doyle, L.A. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology 2021, 78, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Mariño-Enríquez, A.; Wang, W.L.; Roy, A.; Lopez-Terrada, D.; Lazar, A.J.; Fletcher, C.D.; Coffin, C.M.; Hornick, J.L. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am. J. Surg. Pathol. 2011, 35, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Favini, F.; Resti, A.G.; Collini, P.; Casanova, M.; Meazza, C.; Trecate, G.; Ferrari, A. Inflammatory myofibroblastic tumor of the conjunctiva: Response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr. Blood Cancer 2010, 54, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Kube, S.; Vokuhl, C.; Dantonello, T.; Scheer, M.; Hallmen, E.; Feuchtgruber, S.; Escherich, G.; Niggli, F.; Kuehnle, I.; von Kalle, T.; et al. Inflammatory myofibroblastic tumors—A retrospective analysis of the Cooperative Weichteilsarkom Studiengruppe. Pediatric Blood Cancer 2018, 65, e27012. [Google Scholar] [CrossRef] [PubMed]
- Theilen, T.-M.; Soerensen, J.; Bochennek, K.; Becker, M.; Schwabe, D.; Rolle, U.; Klingebiel, T.; Lehrnbecher, T. Crizotinib in ALK(+) inflammatory myofibroblastic tumors-Current experience and future perspectives. Pediatr. Blood Cancer 2018, 65, e26920. [Google Scholar] [CrossRef] [PubMed]
- Lovly, C.M.; Gupta, A.; Lipson, D.; Otto, G.; Brennan, T.; Chung, C.T.; Borinstein, S.C.; Ross, J.S.; Stephens, P.J.; Miller, V.A.; et al. Inflammatory Myofibroblastic Tumors Harbor Multiple Potentially Actionable Kinase Fusions. Cancer Discov. 2014, 4, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M.; et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, B.; Palmer, R.H. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat. Rev. Cancer 2013, 13, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P. Anaplastic Lymphoma Kinase as a Cancer Target in Pediatric Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, M.; Brennan, B.; Alaggio, R.; Kelsey, A.; Orbach, D.; van Noesel, M.M.; Corradini, N.; Minard-Colin, V.; Zanetti, I.; Bisogno, G.; et al. Inflammatory myofibroblastic tumor: The experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur. J. Cancer 2020, 127, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Baldi, G.G.; Brahmi, M.; Lo Vullo, S.; Cojocaru, E.; Mir, O.; Casanova, M.; Vincenzi, B.; De Pas, T.M.; Grignani, G.; Pantaleo, M.A.; et al. The Activity of Chemotherapy in Inflammatory Myofibroblastic Tumors: A Multicenter, European Retrospective Case Series Analysis. Oncologist 2020, 25, e1777–e1784. [Google Scholar] [CrossRef]
- Butrynski, J.E.; D’Adamo, D.R.; Hornick, J.L.; Dal Cin, P.; Antonescu, C.R.; Jhanwar, S.C.; Jhanwar, S.C.; Ladanyi, M.; Capelletti, M.; Rodig, S.J.; et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010, 363, 1727–1733. [Google Scholar] [CrossRef] [Green Version]
- Mossé, Y.P.; Voss, S.D.; Lim, M.S.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK with Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3215–3221. [Google Scholar] [CrossRef]
- Michels, S.Y.F.; Scheel, A.H.; Wündisch, T.; Heuckmann, J.M.; Menon, R.; Puesken, M.; Kobe, C.; Pasternack, H.; Heydt, C.; Scheffler, M.; et al. ALK(G1269A) mutation as a potential mechanism of acquired resistance to crizotinib in an ALK-rearranged inflammatory myofibroblastic tumor. NPJ Precis. Oncol. 2017, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansfield, A.S.; Murphy, S.J.; Harris, F.R.; Robinson, S.I.; Marks, R.S.; Johnson, S.H.; Smadbeck, J.B.; Halling, G.C.; Yi, E.S.; Wigle, D.; et al. Chromoplectic TPM3–ALK rearrangement in a patient with inflammatory myofibroblastic tumor who responded to ceritinib after progression on crizotinib. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Farris, N.; Sampson, M. Single-agent rituximab for treatment of multifocal and multiple relapsed pulmonary inflammatory myofibroblastic tumor in an adolescent patient. Pediatr. Blood Cancer 2021, 68, e29131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).