Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Study Population
3.2. Clinical Outcomes
3.3. Predictors of Disease Severity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCloskey, B.; Heymann, D.L. SARS to novel coronavirus—Old lessons and new lessons. Epidemiol. Infect. 2020, 148, e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report. Available online: https://covid19.who.int/ (accessed on 1 October 2021).
- Noh, J.Y.; Yoon, J.G.; Seong, H.; Choi, W.S.; Sohn, J.W.; Cheong, H.J.; Kim, W.J.; Song, J.Y. Asymptomatic infection and atypical manifestations of COVID-19: Comparison of viral shedding duration. J. Infect. 2020, 81, 816–846. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Yoon, J.G.; Seong, H.; Choi, W.S.; Sohn, J.W.; Cheong, H.J.; Kim, W.J.; Song, J.Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coro-na- virus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 1–11. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Li, D.; Wang, X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Alsohime, F.; Temsah, M.-H.; Al-Nemri, A.M.; Somily, A.M.; Al-Subaie, S. COVID-19 infection prevalence in pediatric population: Etiology, clinical presentation, and outcome. J. Infect. Public Heal. 2020, 13, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.M.; Fu, J.F.; Shu, Q.; Chen, Y.H.; Hua, C.Z.; Li, F.B.; Lin, R.; Tang, L.-F.; Wand, T.-L.; Wand, W.; et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020, 16. [Google Scholar] [CrossRef] [Green Version]
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef]
- Gonzalez-Dambrauskas, S.; Vasquez-Hoyos, P.; Camporesi, A.; Cantillano, E.M.; Dallefeld, S.; Dominguez-Rojas, J.; Francoeur, C.; Gurbanov, A.; Vega, L.M.; Shein, S. Adriana Yock-Corrales, ToddKarsies. medRxiv 2021. [Google Scholar] [CrossRef]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2020, 38, 51–57. [Google Scholar] [CrossRef]
- Yasuhara, J.; Kuno, T.; Takagi, H.; Sumitomo, N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr. Pulmonol. 2020, 55, 2565–2575. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, F.; Wang, C.; Wu, J.; Liu, J.; Chen, X.; Xiao, H.; Liu, Z.; Wu, Z.; Lu, X.; et al. Children Hospitalized with Severe COVID-19 in Wuhan. Pediatr. Infect. Dis. J. 2020, 39, e91–e94. [Google Scholar] [CrossRef]
- World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: Scientific brief. 15 May 2020. WHO/2019-nCoV/Sci_Brief/Multisystem_Syndrome_Children/2020.1. Available online: https://apps.who.int/iris/handle/10665/332095 (accessed on 1 October 2021).
- Yock-Corrales, A.; Lenzi, J.; Brizuela, M.; Valentini, P.; Buonsenso, D.; COVID-DOMINGO Study Group. Tackling antibiotic resistance during the COVID-19 pandemic is a new challenge for paediatricians. Acta Paediatr. 2021, 110, 2650–2651. [Google Scholar] [CrossRef]
- Yock-Corrales, A.; Lenzi, J.; Ulloa-Gutiérrez, R.; Gómez-Vargas, J.; Antúnez-Montes, O.Y.; Aida, J.A.R.; del Aguila, O.; Arteaga-Menchaca, E.; Campos, F.; Uribe, F.; et al. Acute Abdomen and Appendicitis in 1010 Pediatric Patients With COVID-19 or MIS-C: A Multinational Experience from Latin America. Pediatr. Infect. Dis. J. 2021, 40, e364–e369. [Google Scholar] [CrossRef]
- Yock-Corrales, A.; Lenzi, J.; Ulloa-Gutiérrez, R.; Gómez-Vargas, J.; Antúnez-Montes, O.Y.; Aida, J.A.R.; del Aguila, O.; Arteaga-Menchaca, E.; Campos, F.; Uribe, F.; et al. High rates of antibiotic prescriptions in children with COVID-19 or multisystem inflammatory syndrome: A multinational experience in 990 cases from Latin America. Acta Paediatr. 2021, 110, 1902–1910. [Google Scholar] [CrossRef]
- Antúnez-Montes, O.Y.; Escamilla, M.I.; Figueroa-Uribe, A.F.; Arteaga-Menchaca, E.; Lavariega-Sárachaga, M.; Salcedo-Lozada, P.; Sunohara, R.A.; Melchior, P.; del Razo, J.O.F.; Tirado-Caballero, J.C.; et al. COVID-19 in South American Children: A Call for Action. Pediatr. Infect. Dis. J. 2020, 39, e332–e334. [Google Scholar] [CrossRef]
- Antúnez-Montes, O.Y.; Escamilla, M.I.; Figueroa-Uribe, A.F.; Arteaga-Menchaca, E.; Lavariega-Saráchaga, M.; Salcedo-Lozada, P.; Melchior, P.; de Oliveira, R.B.; Caballero, J.C.T.; Redondo, H.P.; et al. COVID-19 and Multisystem Inflammatory Syndrome in Latin American Children. Pediatr. Infect. Dis. J. 2020, 40, e1–e6. [Google Scholar] [CrossRef]
- Martin, B.; DeWitt, P.E.; Russell, S.; Anand, A.; Bradwell, K.R.; Bremer, C.; Gabriel, D.; Girvin, A.T.; Hajagos, J.G.; et al. Children with SARS-CoV-2 in the National COVID Cohort Collaborative (N3C). medRxiv 2021. [Google Scholar] [CrossRef]
- Preston, L.E.; Chevinsky, J.R.; Kompaniyets, L.; Lavery, A.M.; Kimball, A.; Boehmer, T.K.; Goodman, A.B. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Netw. Open 2021, 4, e215298. [Google Scholar] [CrossRef]
- Kara, A.A.; Böncüoğlu, E.; Kıymet, E.; Arıkan, K.; Şahinkaya, S.; Düzgöl, M.; Cem, E.; Çelebi, M.; Ağın, H.; Bayram, S.N.; et al. Evaluation of predictors of severe-moderate COVID-19 infections at children: A review of 292 children. J. Med. Virol. 2021, 93, 6634–6640. [Google Scholar] [CrossRef]
- Geva, A.; Patel, M.M.; Newhams, M.M.; Young, C.C.; Son, M.B.F.; Kong, M.; Maddux, A.B.; Hall, M.W.; Riggs, B.J.; Singh, A.R.; et al. Data-driven clustering identifies features distinguishing multisystem inflammatory syndrome from acute COVID-19 in children and adolescents. eClinicalMedicine 2021, 40, 101112. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2021, 59, 2101341. [Google Scholar] [CrossRef]
- Buonsenso, D.; Di Giuda, D.; Sigfrid, L.; Pizzuto, D.A.; Di Sante, G.; De Rose, C.; Lazzareschi, I.; Sali, M.; Baldi, F.; Chieffo, D.P.R.; et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child. Adolesc. Health 2021, 5, 677–680. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Murias, S.; Garde, A.M.-D.; Soriano-Arandes, A.; Pareja, M.; Otheo, E.; Moraleda, C.; Tagarro, A.; Calvo, C. Prevalence of thrombotic complications in children with SARS-CoV-2. Arch. Dis. Child. 2021, 106, 1129–1132. [Google Scholar] [CrossRef]
- Milross, L.; Majo, J.; Cooper, N.; Kaye, P.M.; Bayraktar, O.; Filby, A.; Fisher, A.J. Post-mortem lung tissue: The fossil record of the pathophysiology and immunopathology of severe COVID-19. Lancet Respir. Med. 2021, 10, 95–106. [Google Scholar] [CrossRef]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.J.; Cornwell, M.; Myndzar, K.; Rolling, C.C.; Xia, Y.; Drenkova, K.; Biebuyck, A.; Fields, A.T.; Tawil, M.; Luttrell-Williams, E.; et al. Platelets amplify endotheliopathy in COVID-19. Sci. Adv. 2021, 7, eabh2434. [Google Scholar] [CrossRef]

| Entire Cohort (n = 316) | PICU (n = 26) | Not PICU (n = 290) | p Value | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age, years (median, IQR) | 3.93 (0.62–10.7) | 8 (4.27–12.22) | 3.64 (0.43–10.58) | 0.005 |
| Sex | 0.7 | |||
| Male, n (%) | 171 (54.1%) | 15 (57.7%) | 156 (53.8%) | |
| Female, n (%) | 145 (45.9%) | 11 (42.3%) | 134 (46.2%) | |
| Comorbidities, n (%) | 113 (35.8%) | 12 (46.2%) | 101 (34.8%) | 0.24 |
| Signs and symptoms at presentation | ||||
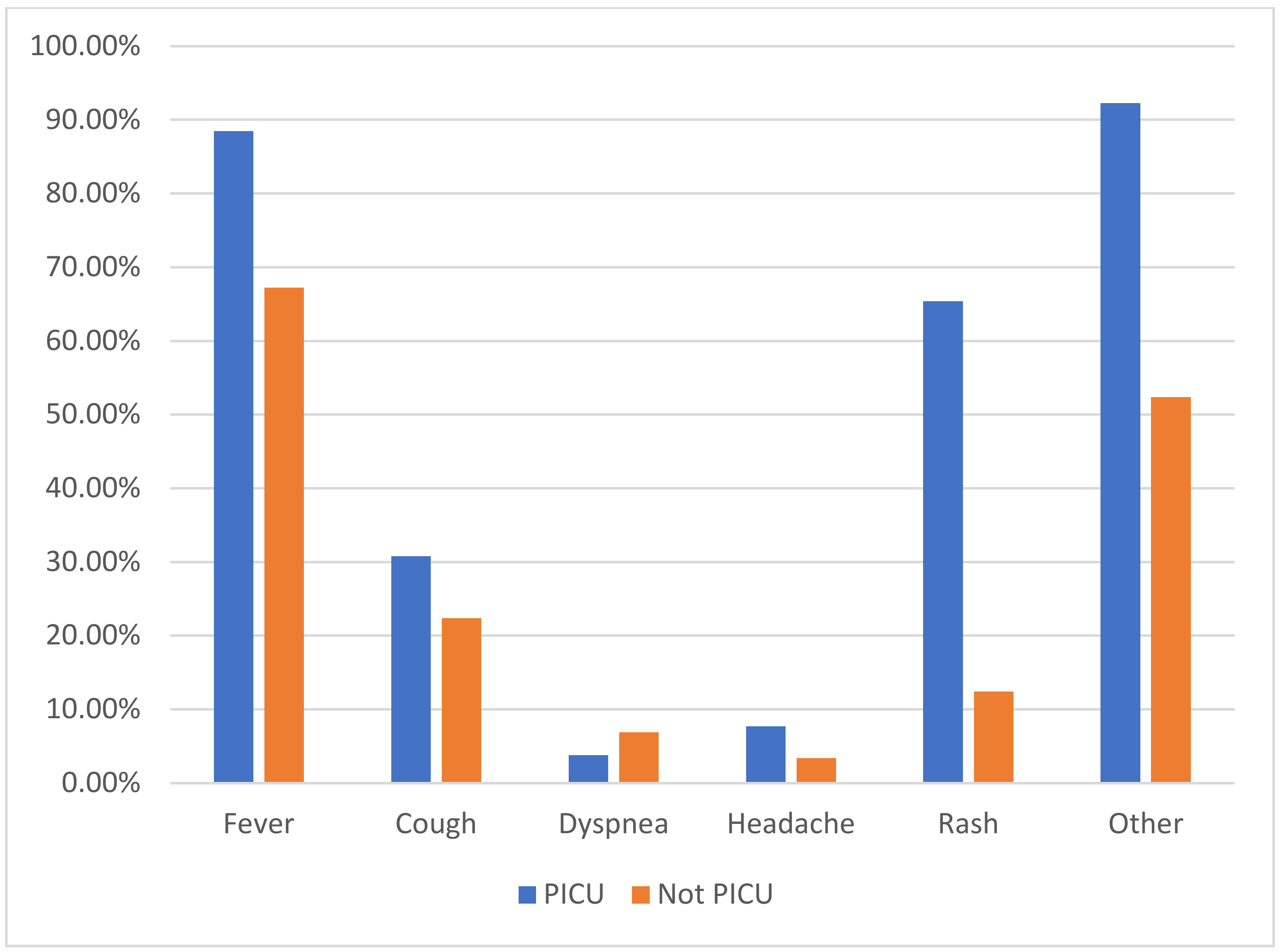
| Fever, n (%) | 218 (69.0%) | 23 (88.5%) | 195 (67.2%) | 0.02 |
| Cough, n (%) | 73 (23.1%) | 8 (30.8%) | 65 (22.4%) | 0.33 |
| Dyspnea, n (%) | 21 (6.6%) | 1 (3.8%) | 20 (6.9%) | 1 |
| Headache, n (%) | 12 (3.8%) | 2 (7.7%) | 10 (3.4%) | 0.25 |
| Rash, n (%) | 53 (16.8%) | 17 (65.4%) | 36 (12.4%) | <0.0001 |
| Other, n (%) | 176 (55.7%) | 24 (92.3%) | 152 (52.4%) | <0.0001 |
| MIS-C, n (%) | 59 (18.7%) | 21 (80.8%) | 38 (13.1%) | <0.0001 |
| Laboratory findings at the diagnosis | ||||
| D-dimer dosage, n (%) | 240 (75.9%) | 24 (92.3%) | 216 (74.5%) | 0.05 |
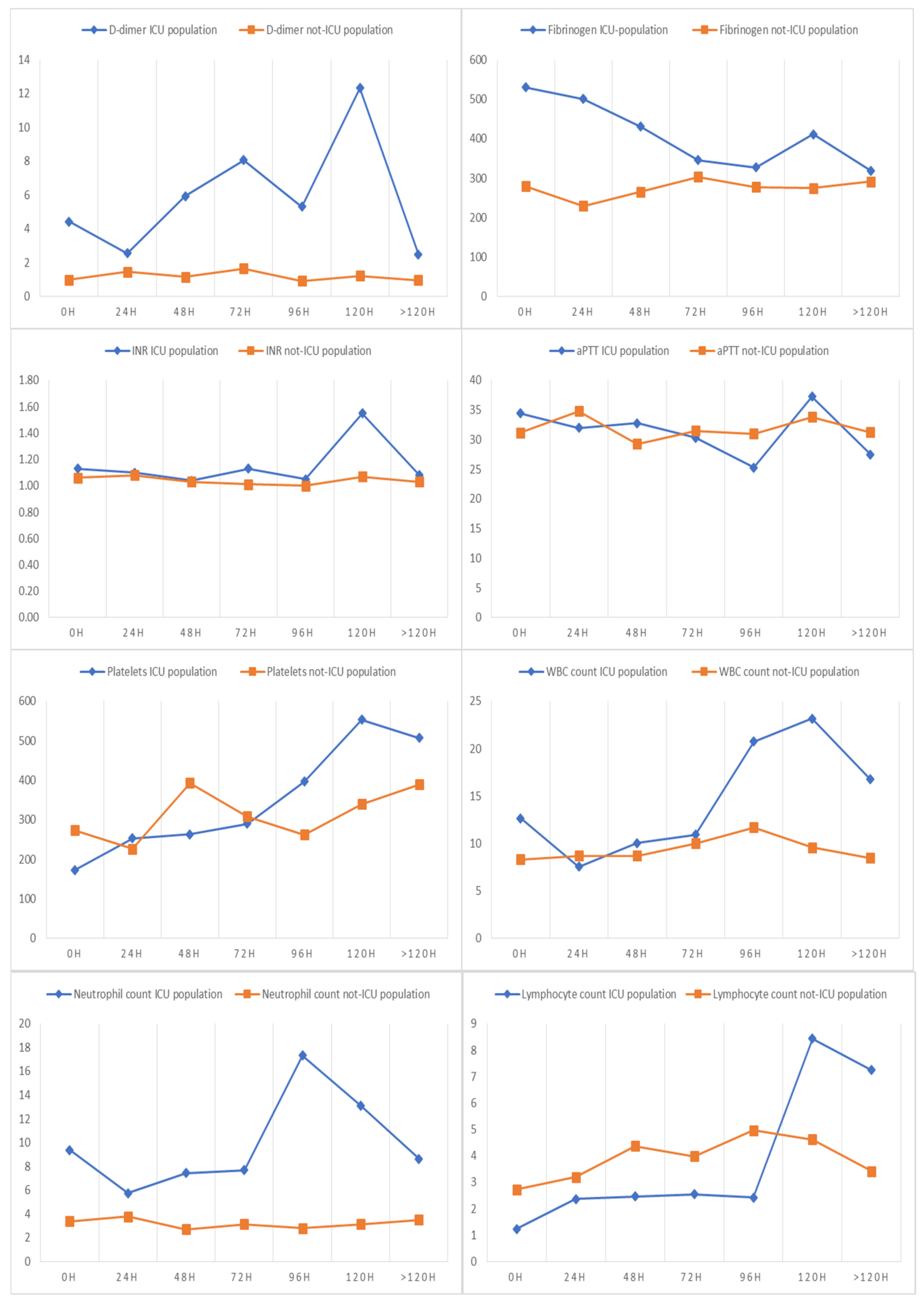
| D-dimer (median, IQR) | 1.06 (0.43–2.55) | 4.55 (3.38–8.29) | 0.99 (0.41–1.78) | <0.0001 |
| D-dimer altered, n (%) | 169 (70.4%) | 24 (100%) | 145 (67.1%) | 0.001 |
| Fibrinogen dosage, n (%) | 260 (82.3%) | 26 (100%) | 234 (80.7%) | 0.007 |
| Fibrinogen (median, IQR) | 286 (233–406) | 532 (327–631) | 290 (233–438) | <0.0001 |
| Hyperfibrinogenemia, n (%) | 65 (25%) | 16 (61.5%) | 49 (20.9%) | <0.0001 |
| Hypofibrinogenemia, n (%) | 28 (10.8%) | 3 (11.5%) | 25 (10.7%) | 0.89 |
| Fibrinogen altered, n (%) | 93 (35.8%) | 19 (73%) | 74 (31.6%) | <0.0001 |
| INR and aPTT dosage, n (%) | 236 (74.7%) | 26 (100%) | 210 (72.4%) | 0.001 |
| INR (median, IQR) | 1.08 (1–1.17) | 1.22 (1.09–1.35) | 1.06 (0.98–1.17) | 0.003 |
| aPTT, sec (median, IQR) | 31.35 (27.58–35) | 34 (28.84–42.55) | 31.05 (27.4–34.9) | 0.31 |
| Platelet count, n (%) | 272 (86.1%) | 25 (96.2%) | 247 (85.2%) | 0.15 |
| Platelet count ×109 per L (median, IQR) | 268.5 (197.0–345.0) | 150.0 (81.0–225.5) | 268.5 (205.5–350.5) | 0.001 |
| Platelet count altered, n (%) | 63 (23.2%) | 11 (44.0%) | 52 (21.1%) | 0.01 |
| Thrombocytopenia, n (%) | 39 (14.3%) | 9 (36%) | 30 (12.1%) | 0.001 |
| Thrombocytosis, n (%) | 24 (8.8%) | 2 (8%) | 22 (8.9%) | 1 |
| Blood count, n (%) | 253 (80.1%) | 20 (76.9%) | 233 (80.3%) | 0.67 |
| White blood cell count, ×109 per L (median, IQR) | 8.46 (6.14–12.07) | 13.1 (6.4–16.1) | 7.9 (5.7–11.5) | 0.022 |
| Neutrophil count, ×109 per L (median, IQR) | 3.59 (1.79–6.87) | 9.4 (5.2–13.0) | 3.5 (1.7–6.7) | <0.0001 |
| Lymphocyte count, ×109 per L (median, IQR) | 2.59 (1.57–4.64) | 1.1 (0.6–1.8) | 2.5 (1.5–4.6) | <0.0001 |
| Entire Cohort (n = 316) | PICU (n = 26) | Not PICU (n = 290) | p Value | |
|---|---|---|---|---|
| Treatments | ||||
| Clexane, n (%) | 32 (10.2%) | 13 (50%) | 19 (6.6%) | <0.0001 |
| Aspirin, n (%) | 48 (15.2%) | 16 (61.5%) | 32 (11.0%) | <0.0001 |
| IVIG, n (%) | 56 (17.7%) | 20 (76.9%) | 36 (12.4%) | <0.0001 |
| Outcomes | ||||
| Intubated, n (%) | 14 (4.8%) | 14 (56.0%) | 0 (0%) | <0.0001 |
| Not invasive ventilation, n (%) | 5 (1.8%) | 3 (17.6%) | 2 (0.8%) | 0.002 |
| Myocardial disfunction, n (%) | 20 (6.3%) | 14 (53.8%) | 6 (2.1%) | <0.0001 |
| Coronary anomalies, n (%) | 8 (2.5%) | 4 (15.4%) | 4 (1.4%) | 0.002 |
| Embolism, n (%) | 3 (0.9%) | 2 (7.7%) | 1 (0.3%) | 0.019 |
| Hemorrhages, n (%) | 2 (0.6%) | 2 (7.7%) | 0 (0%) | 0.007 |
| Sequelae *, n (%) | 13 (4.1%) | 5 (20.0%) | 8 (2.8%) | <0.0001 |
| Dead, n (%) | 3 (0.9%) | 1 (3.8%) | 2 (0.7%) | 0.22 |
| Univariable OR (95% CI) | p Value | Multivariable OR (95% CI) | p Value | Multivariable OR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Demographics and clinical characteristics | ||||||
| Age, years * | 1.56 (1.06–2.3) | 0.02 | 1.1 (0.45–2.65) | 0.83 | ||
| Male sex (vs. female) | 1.1 (0.52–2.63) | 0.7 | ||||
| MIS-C (vs. not-MIS-C) | 27.85 (9.91–78.27) | <0.0001 | ||||
| Comorbidities present (vs. not present) | 1.6 (0.71–3.6) | 0.25 | ||||
| Asymptomatic present (vs. not present) | 0.28 (0.04–2.15) | 0.22 | ||||
| Fever present (vs. not present) | 3.73 (1.09–12.75) | 0.03 | 0.95 (0.17–5.14) | 0.95 | ||
| Cough present (vs. not present) | 1.54 (0.64–3.7) | 0.33 | 1.06 (0.37–3.07) | 0.9 | ||
| Dyspnea present (vs. not present) | 0.54 (0.07–4.19) | 0.57 | 1.38 (0.13–14.28) | 0.79 | ||
| Headache present (vs. not present) | 2.33 (0.48–11.26) | 0.29 | 1.92 (0.27–13.65) | 0.51 | ||
| Rash present (vs. not present) | 13.3 (5.52–32.13) | <0.0001 | 10.7 (3.5–32.93) | <0.0001 | ||
| Other symptoms 1 present (vs. not present) | 10.9 (2.52–46.95) | 0.001 | ||||
| Laboratory findings | ||||||
| D-dimer, mcg/mL * | 2.67 (1.68–4.23) | <0.0001 | 1.9 (1.11–3.25) | 0.02 | 2.08 (1.36–3.19) | 0.001 |
| Fibrinogen, mg/dL * | 1.97 (1.4–2.8) | <0.0001 | 1.09 (0.65–1.82) | 0.74 | ||
| Hyperfibrinogenemia present (vs. not present) | 6.04 (2.58–14.14) | <0.0001 | ||||
| INR * | 1.51 (1.07–2.12) | 0.02 | 1.24 (0.83–1.85) | 0.3 | ||
| aPTT, sec * | 1.71 (1.21–2.43) | 0.002 | 0.95 (0.58–1.57) | 0.85 | ||
| Platelet count * | 0.47 (0.28–0.77) | 0.003 | 0.6 (0.26–1.37) | 0.22 | ||
| Thrombocytopenia present (vs. not present) | 4.07 (1.65–10.02) | 0.002 | ||||
| White blood cell count * | 1.64 (1.13–2.37) | 0.008 | 1.19 (0.13–10.48) | 0.88 | ||
| Neutrophil count * | 1.98 (1.4–2.8) | <0.0001 | 1.32 (0.15–11.7) | 0.8 | ||
| Lymphocyte count * | 0.08 (0.02–0.33) | <0.0001 | 0.21 (0.02–1.77) | 0.15 |
| Univariable OR (95% CI) | p Value | Multivariable OR (95% CI) | p Value | |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| MIS-C (vs. not-MIS-C) | 27.85 (9.91–78.27) | <0.0001 | 12.42 (2.3–66.9) | 0.003 |
| Rash present (vs. not present) | 13.3 (5.52–32.13) | <0.0001 | 2.27 (0.57–8.99) | 0.241 |
| Laboratory findings | ||||
| D-dimer, mcg/mL * | 2.67 (1.68–4.23) | <0.0001 | 1.61 (1.00–2.59) | 0.046 |
| Univariable OR (95% CI) | p Value | |
|---|---|---|
| PICU admission present (vs. not present) | 1.36 (1.17–1.58) | <0.001 |
| Embolism present (vs. not present) | 1.11 (0.95–1.29) | 1.29 |
| Hemorrhages present (vs. not present) | 1.14 (0.98–1.32) | 0.07 |
| Intubation present (vs. not present) | 1.24 (1.08–1.43) | 0.002 |
| Non-invasive ventilation present (vs. not present) | 1.13 (0.99–1.29) | 0.05 |
| Coronary anomalies present (vs. not present) | 1.26 (1.093–1.47) | 0.002 |
| Myocardial dysfunction present (vs. not present) | 1.27 (1.11–1.46) | <0.001 |
| Sequelae (vs. not present) | 1.12 (1.01–1.24) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonsenso, D.; Mariani, F.; Pierri, L.; Morello, R.; Yock-Corrales, A.; Del Aguila, O.; Lazzareschi, I.; Zampino, G.; Nunziata, F.; Valentini, P.; et al. Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study. Children 2022, 9, 279. https://doi.org/10.3390/children9020279
Buonsenso D, Mariani F, Pierri L, Morello R, Yock-Corrales A, Del Aguila O, Lazzareschi I, Zampino G, Nunziata F, Valentini P, et al. Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study. Children. 2022; 9(2):279. https://doi.org/10.3390/children9020279
Chicago/Turabian StyleBuonsenso, Danilo, Francesco Mariani, Luca Pierri, Rosa Morello, Adriana Yock-Corrales, Olguita Del Aguila, Ilaria Lazzareschi, Giuseppe Zampino, Francesco Nunziata, Piero Valentini, and et al. 2022. "Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study" Children 9, no. 2: 279. https://doi.org/10.3390/children9020279
APA StyleBuonsenso, D., Mariani, F., Pierri, L., Morello, R., Yock-Corrales, A., Del Aguila, O., Lazzareschi, I., Zampino, G., Nunziata, F., Valentini, P., & Lo Vecchio, A. (2022). Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study. Children, 9(2), 279. https://doi.org/10.3390/children9020279









