Fractures in Osteogenesis Imperfecta: Pathogenesis, Treatment, Rehabilitation and Prevention
Abstract
:1. Introduction
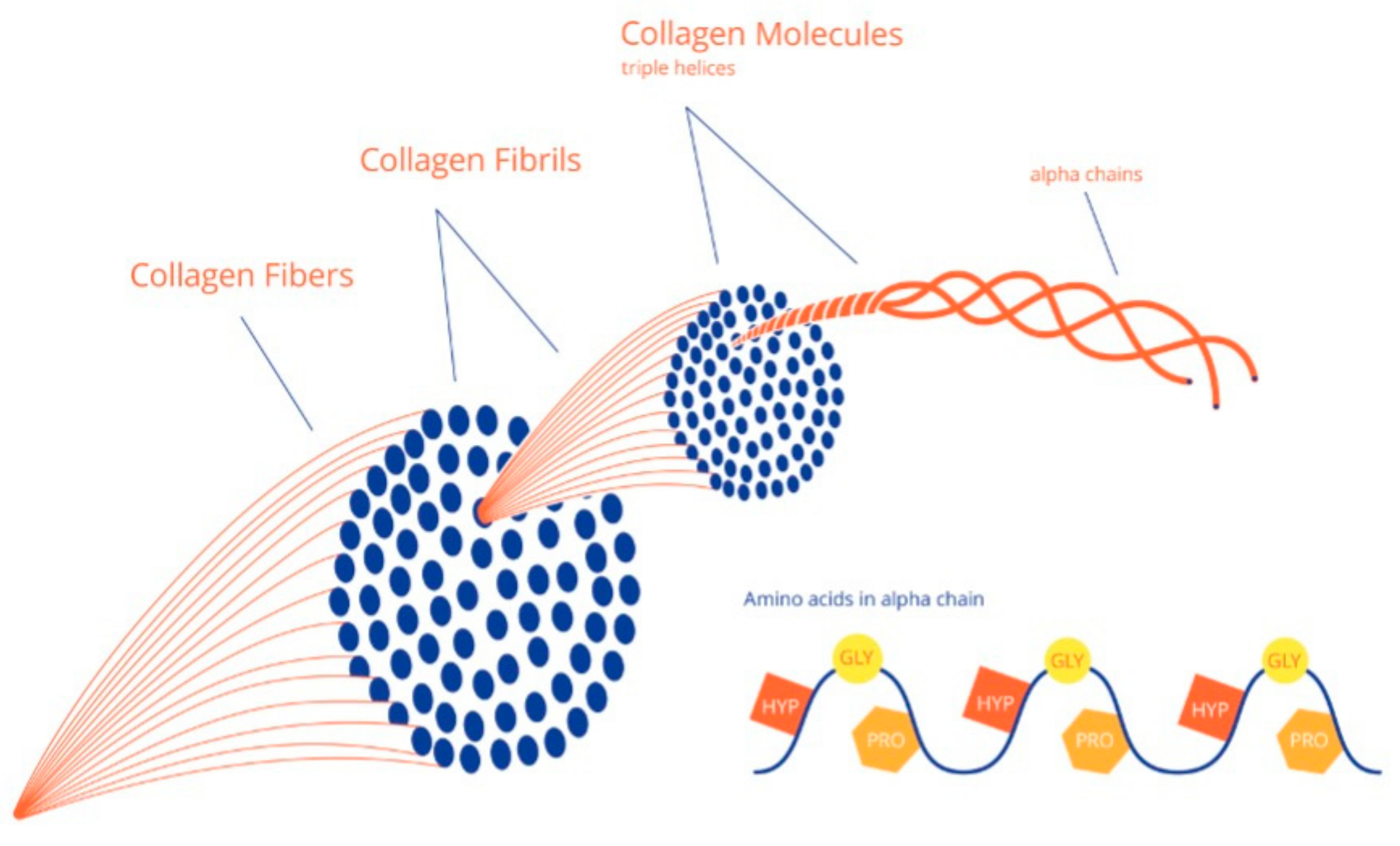
2. Collagen and Bone Tissue Formation
3. Bone Strength and Elasticity in OI
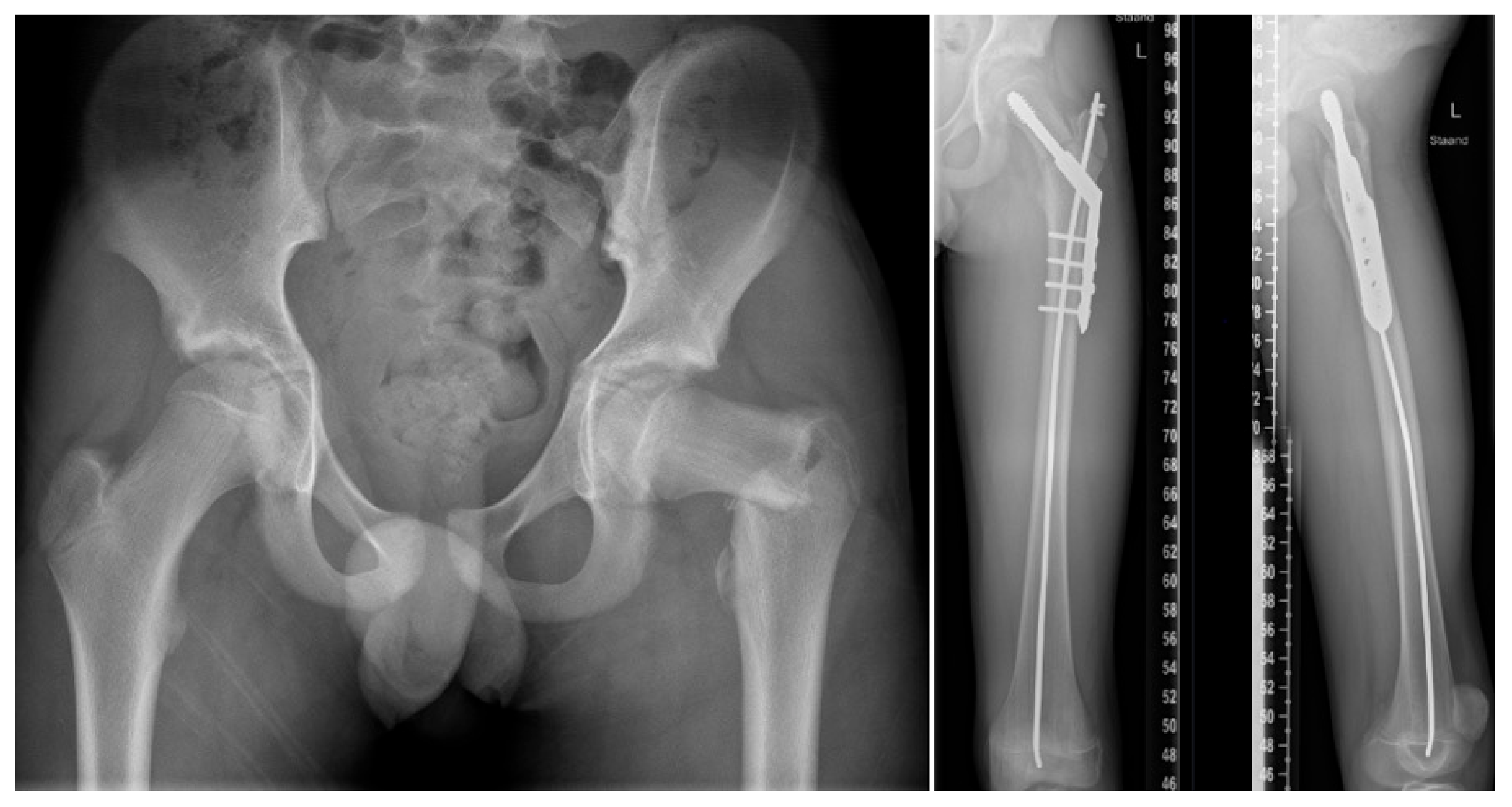
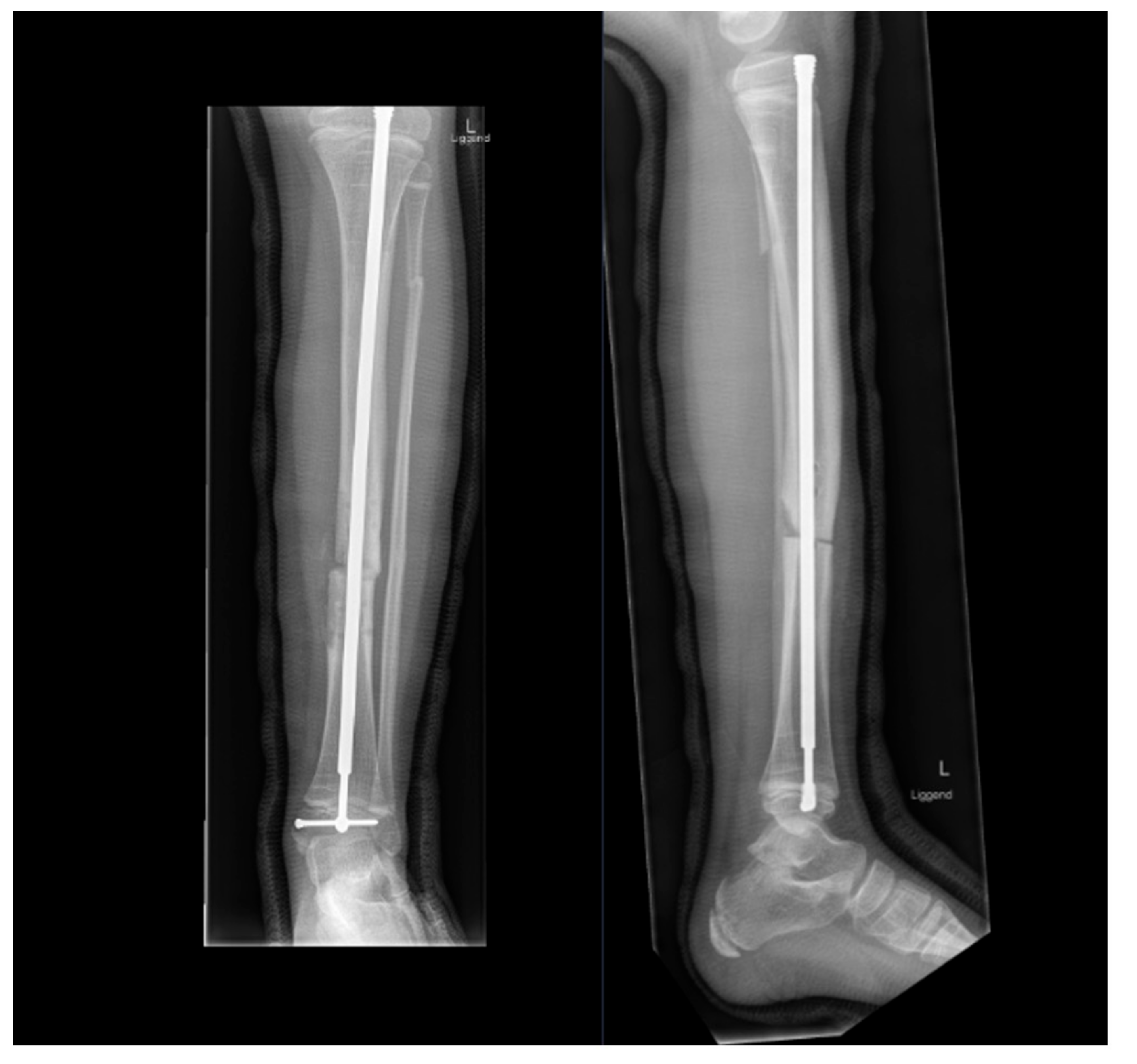
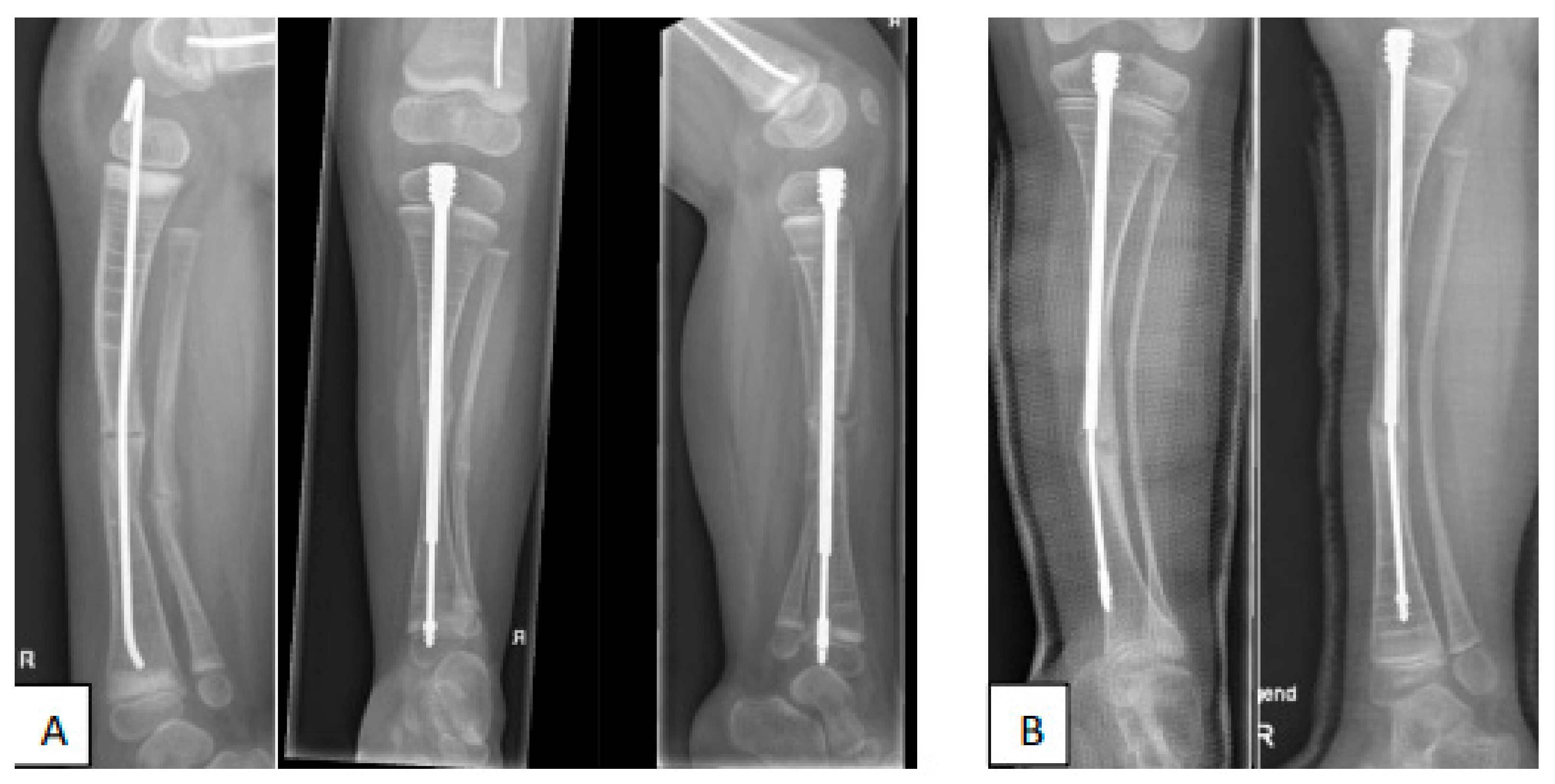
4. Fracture Management
5. Prevention of Fractures
5.1. Medication
5.2. Physical Activity
5.3. Preventive Surgery
5.4. Rehabilitation
6. Multidisciplinary Treatment Challenges
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Botor, M.; Fus-Kujawa, A.; Uroczynska, M. Osteogenesis Imperfecta: Current and Prospective Therapies. Biomolecules 2021, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.S.; Sillence, D.O. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. A 2014, 164A, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Kivirikko, K.I. Collagens and their abnormalities in a wide spectrum of diseases. Annu. Med. 1993, 25, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.H.; Steiner, R.D. Osteogenesis imperfecta. Annu. Rev. Med. 1992, 43, 269–282. [Google Scholar] [CrossRef]
- Prockop, D.J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J. Biol. Chem. 1990, 265, 15349–15352. [Google Scholar] [CrossRef]
- Gao, H.; Ji, B.; Jager, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [Green Version]
- Jäger, I.; Fratzl, P. Mineralized collagen fibrils: A mechanical model with a staggered arrangement of mineral particles. Biophys. J. 2000, 79, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Nijhuis, W.H.; Eastwood, D.M.; Allgrove, J.; Hvid, I.; Weinans, H.H.; Bank, R.A.; Sakkers, R.J. Current concepts in osteogenesis imperfecta: Bone structure, biomechanics and medical management. J. Child Orthop. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Jones, S.J.; Glorieux, F.H.; Travers, R.; Boyde, A. The microscopic structure of bone in normal children and patients with osteogenesis imperfecta: A survey using backscattered electron imaging. Calcif. Tissue Int. 1999, 64, 8–17. [Google Scholar] [CrossRef]
- Yeni, Y.N.; Brown, C.U.; Wang, Z.; Norman, T.L. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 1997, 21, 453–459. [Google Scholar] [CrossRef]
- Nishiyama, K.K.; Boyd, S.K. In vivo assessment of trabecular and cortical bone microstructure. Clin. Calcium 2011, 21, 1011–1019. [Google Scholar] [PubMed]
- Kocijan, R.; Muschitz, C.; Haschka, J. Bone structure assessed by HR-pQCT, TBS and DXL in adult patients with different types of osteogenesis imperfecta. Osteoporos. Int. 2015, 26, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; McCarthy, E.F.; Rossiter, K. The effect of intravenous pamidronate on bone mineral density, bone histomorphometry, and parameters of bone turnover in adults with type IA osteogenesis imperfecta. Calcif. Tissue Int. 2003, 72, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Travers, R.; Plotkin, H.; Glorieux, F.H. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J. Clin. Investig. 2002, 110, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Boutroy, S.; Hans, D.; Sornay-Rendu, E. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: The OFELY study. Osteoporos. Int. 2013, 24, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Winzenberg, T.M.; Powell, S.; Shaw, K.A.; Jones, G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst. Rev. 2010, 10, e23475. [Google Scholar] [CrossRef]
- Wilsford, L.D.; Sullivan, E.; Mazur, L.J. Risk factors for vitamin D deficiency in children with osteogenesis imperfecta. J. Pediatr. Orthop. 2013, 33, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Sakkers, R.; Kok, D.; Engelbert, R.; van Dongen, A.; Jansen, M.; Pruijs, H.; Verbout, A.; Schweitzer, D.; Uiterwaal, C. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: A 2-year randomised placebo-controlled study. Lancet 2004, 363, 1427–1431. [Google Scholar] [CrossRef]
- Scheres, L.J.J.; van Dijk, F.S.; Harsevoort, A.J.; van Dijk, A.T.H.; Dommisse, A.M.; Janus, G.J.M.; Franken, A.A.M. Adults with osteogenesis imperfecta: Clinical characteristics of 151 patients with a focus on bisphosphonate use and bone density measurements. Bone Rep. 2018, 8, 168–172. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Zebaze, R.M.; Ghasem-Zadeh, A.; Iuliano-Burns, S.; Hardidge, A.; Seeman, E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone 2013, 52, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Götherström, C.; David, A.L.; Walther-Jallow, L.; Åström, E.; Westgren, M. Mesenchymal Stem Cell Therapy for Osteogenesis Imperfecta. Clin. Obstet. Gynecol. 2021, 64, 898–903. [Google Scholar] [CrossRef]
- Glorieux, F.H.; Devogelaer, J.P.; Durigova, M. BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: Results of a randomized phase 2a trial. J. Bone Miner. Res. 2017, 32, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veilleux, L.N.; Rauch, F. Muscle-bone interactions in pediatric bone diseases. Curr. Osteoporos. Rep. 2017, 15, .425–432. [Google Scholar] [CrossRef]
- Nijhuis, W.; Franken, A.; Ayers, K.; Damas, C.; Folkestad, L.; Forlino, A.; Fraschini, P.; Hill, C.; Janus, G.; Kruse, R.; et al. A standard set of outcome measures for the comprehensive assessment of osteogenesis imperfecta. Orphanet. J. Rare Dis. 2021, 2021, 16140. [Google Scholar] [CrossRef]
- Azzam, K.A.; Rush, E.T.; Burke, B.R.; Nabower, A.M.; Esposito, P.W. Mid-term Results of Femoral and Tibial Osteotomies and Fassier-Duval Nailing in Children With Osteogenesis Imperfecta. J. Pediatr. Orthop. 2018, 38, 331–336. [Google Scholar] [CrossRef]
- Sakkers, R.J.; Montpetit, K.; Tsimicalis, A.; Wirth, T.; Verhoef, M.; Hamdy, R.; Ouellet, J.A.; Castelein, R.M.; Damas, C.; Janus, G.J.; et al. A roadmap to surgery in osteogenesis imperfecta: Results of an international collaboration of patient organizations and interdisciplinary care teams. Acta Orthop. 2021, 92, 608–614. [Google Scholar] [CrossRef]
- Mueller, B.; Engelbert, R.; Baratta-Ziska, F.; Bartels, B.; Blanc, N.; Brizola, E.; Fraschini, P.; Hill, C.; Marr, C.; Mills, L.; et al. Consensus statement on physical rehabilitation in children and adolescents with osteogenesis imperfecta. Orphanet. J. Rare Dis. 2018, 13, 158. [Google Scholar] [CrossRef] [Green Version]
- Veilleux, L.N.; Pouliot-Laforte, A.; Lemay, M.; Cheung, M.S.; Glorieux, F.H.; Rauch, F. The functional muscle-bone unit in patients with osteogenesis imperfecta type I. Bone 2015, 79, 52–57. [Google Scholar] [CrossRef]
- Tsimicalis, A.; Denis-Larocque, G.; Michalovic, A.; Lepage, C.; Williams, K.; Yao, T.R.; Palomo, T.; Dahan-Oliel, N.; Le May, S.; Rauch, F. The psychosocial experience of individuals living with osteogenesis imperfecta: A mixed-methods systematic review. Qual. Life Res. 2016, 25, 1877–1896. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijhuis, W.; Verhoef, M.; van Bergen, C.; Weinans, H.; Sakkers, R. Fractures in Osteogenesis Imperfecta: Pathogenesis, Treatment, Rehabilitation and Prevention. Children 2022, 9, 268. https://doi.org/10.3390/children9020268
Nijhuis W, Verhoef M, van Bergen C, Weinans H, Sakkers R. Fractures in Osteogenesis Imperfecta: Pathogenesis, Treatment, Rehabilitation and Prevention. Children. 2022; 9(2):268. https://doi.org/10.3390/children9020268
Chicago/Turabian StyleNijhuis, Wouter, Marjolein Verhoef, Christiaan van Bergen, Harrie Weinans, and Ralph Sakkers. 2022. "Fractures in Osteogenesis Imperfecta: Pathogenesis, Treatment, Rehabilitation and Prevention" Children 9, no. 2: 268. https://doi.org/10.3390/children9020268
APA StyleNijhuis, W., Verhoef, M., van Bergen, C., Weinans, H., & Sakkers, R. (2022). Fractures in Osteogenesis Imperfecta: Pathogenesis, Treatment, Rehabilitation and Prevention. Children, 9(2), 268. https://doi.org/10.3390/children9020268







