Feasibility of Real-Time Central Surgical Review for Patients with Advanced-Stage Hepatoblastoma in the JPLT3 Trial
Abstract
:1. Introduction
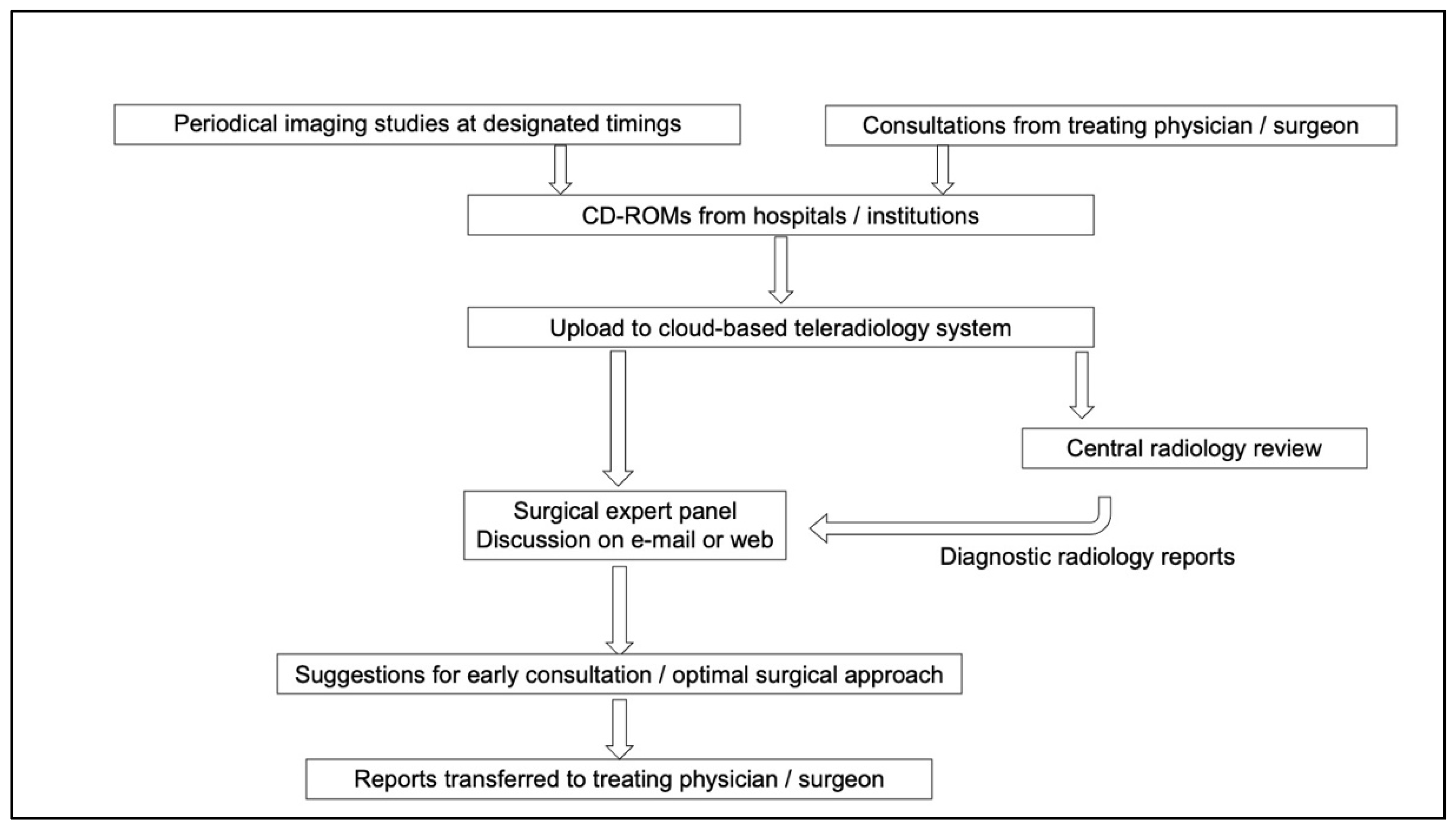
2. Materials and Methods
3. Results
3.1. Surgical Reviews and Responses to Consultations
3.2. Timing of Review Sessions
3.3. Promptness of Review Process
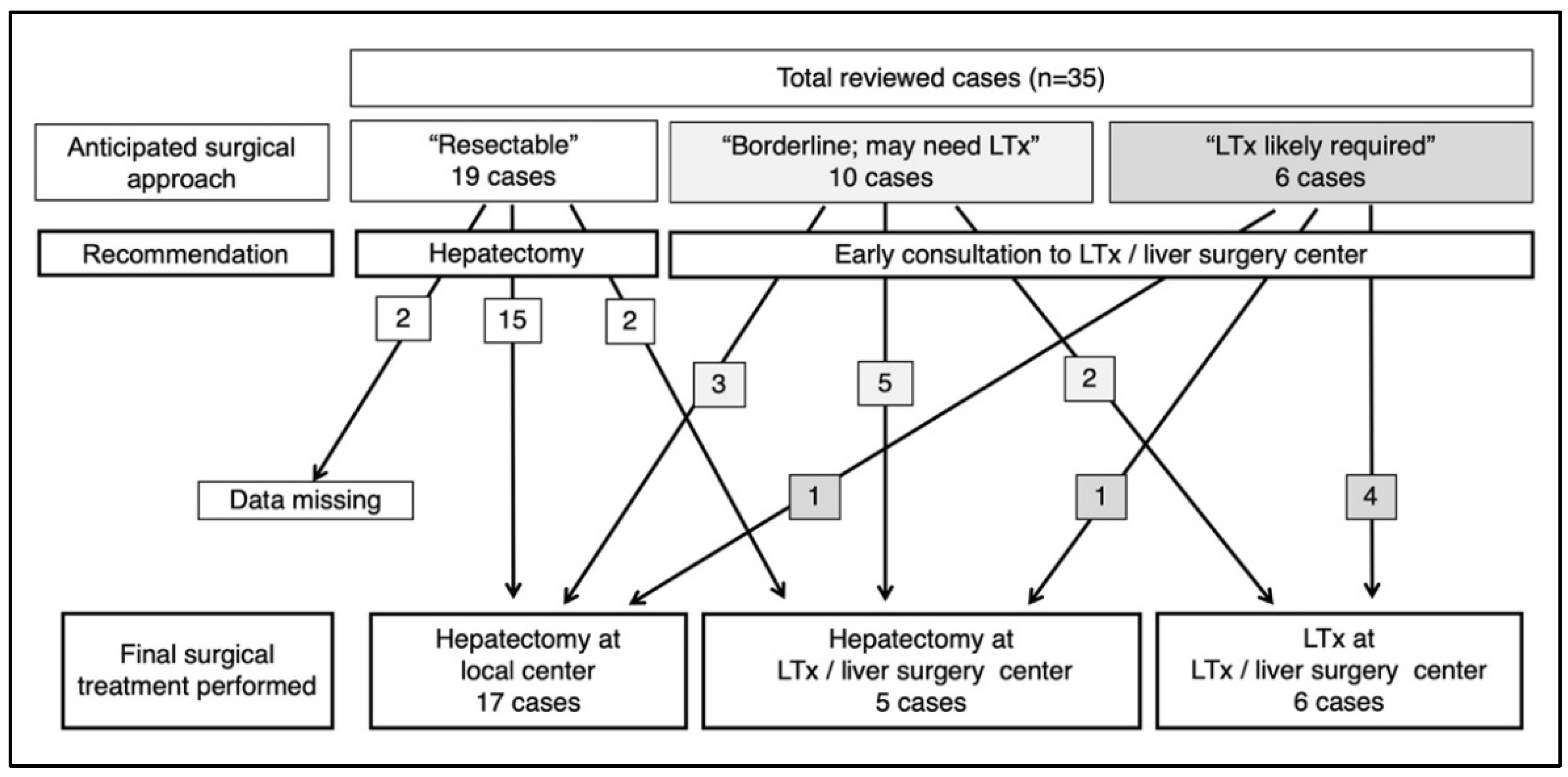
3.4. Recommendations and Surgical Outcome/Patient Referral
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finegold, M.J.; Egler, R.A.; Goss, J.A.; Guillerman, R.P.; Karpen, S.J.; Krishnamurthy, R.; O’Mahony, C.A. Liver tumors: Pediatric population. Liver Transpl. 2008, 14, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Perilongo, G.; Malogolowkin, M.; Feusner, J. Hepatoblastoma clinical research: Lessons learned and future challenges. Pediatr. Blood Cancer 2012, 59, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Hishiki, T.; Watanabe, K.; Ida, K.; Ueda, Y.; Kurihara, S.; Yano, M.; Hoshino, K.; Yokoi, A.; Takama, Y.; et al. Outcome and late complications of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor 2 protocol. J. Clin. Oncol. 2020, 38, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Zsíros, J.; Brugières, L.; Brock, P.; Roebuck, D.; Maibach, R.; Zimmermann, A.; Childs, M.; Pariente, D.; Laithier, V.; International Childhood Liver Tumours Strategy Group (SIOPEL); et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol. 2013, 14, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Zsíros, J.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Czauderna, P.; Roebuck, D.; Childs, M.; Zimmermann, A.; Laithier, V.; et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR study. J. Clin. Oncol. 2010, 28, 2584–2590. [Google Scholar] [CrossRef]
- Katzenstein, H.M.; Langham, M.R.; Malogolowkin, M.H.; Krailo, M.D.; Towbin, A.J.; McCarville, M.B.; Finegold, M.J.; Ranganathan, S.; Dunn, S.; McGahren, E.D.; et al. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): A Children’s Oncology Group, multicentre, phase 3 trial. Lancet Oncol. 2019, 20, 719–727. [Google Scholar] [CrossRef]
- Hishiki, T. Current therapeutic strategies for childhood hepatic tumors: Surgical and interventional treatments for hepatoblastoma. Int. J. Clin. Oncol. 2013, 18, 962–968. [Google Scholar] [CrossRef]
- Lake, C.M.; Tiao, G.M.; Bondoc, A.J. Surgical management of locally-advanced and metastatic hepatoblastoma. Semin. Pediatr. Surg. 2019, 28, 150856. [Google Scholar] [CrossRef]
- Meyers, R.L.; Tiao, G.; de Ville de Goyet, J.; Superina, R.; Aronson, D.C. Hepatoblastoma state of the art: Pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr. Opin. Pediatr. 2014, 26, 29–36. [Google Scholar] [CrossRef]
- Otte, J.B.; de Ville de Goyet, J.; Reding, R. Liver transplantation for hepatoblastoma: Indications and contraindications in the modern era. Pediatr. Transplant. 2005, 9, 557–565. [Google Scholar] [CrossRef]
- Hishiki, T.; Matsunaga, T.; Sasaki, F.; Yano, M.; Ida, K.; Horie, H.; Kondo, S.; Watanabe, K.-I.; Oue, T.; Tajiri, T.; et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol-2, Report from the JPLT. Pediatr. Surg. Int. 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Watanabe, K.; Mori, M.; Hishiki, T.; Yokoi, A.; Ida, K.; Yano, M.; Fujimura, J.; Nogami, Y.; Iehara, T.; Hoshino, K.; et al. Feasibility of dose-dense cisplatin-based chemotherapy in Japanese children with high-risk hepatoblastoma: Analysis of the JPLT3-H pilot study. Pediatr. Blood Cancer 2021, 69, e29389. [Google Scholar] [CrossRef]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Häberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic Tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Czauderna, P.; Haeberle, B.; Hiyama, E.; Rangaswami, A.; Krailo, M.; Maibach, R.; Rinaldi, E.; Feng, Y.; Aronson, D.; Malogolowkin, M.; et al. The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur. J. Cancer 2015, 52, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Roebuck, D.J.; Aronson, D.; Clapuyt, P.; Czauderna, P.; de Ville de Goyet, J.; Gauthier, F.; Mackinlay, G.; Maibach, R.; McHugh, K.; Olsen, O.E.; et al. 2005 PRETEXT: A revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr. Radiol. 2007, 37, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Perilongo, G.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Morland, B.; de Camargo, B.; Zsiros, J.; Roebuck, D.; Zimmermann, A.; et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N. Engl. J. Med. 2009, 361, 1662–1670. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.; Brown, J.; Shafford, E.; Perilongo, G.; Brock, P.; Dicks-Mireaux, C.; Keeling, J.; Phillips, A.; Vos, A.; Plaschkes, J. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach—Results of the first prospective study of the International Society of Pediatric Oncology. J. Clin. Oncol. 2000, 18, 3819–3828. [Google Scholar] [CrossRef]
- Hasse, G.M. The implications of surgical quality assurance in cancer clinical trials. Cancer 1994, 74 (Suppl. 9), 2630–2637. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Fu, X.; Huang, Q.; Zhang, Y.; Luo, Q.; Xiong, X. SmartWADO: An Extensible WADO Middleware for Regional Medical Image Sharing. J. Digit. Imaging 2015, 28, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Genereaux, B.W.; Dennison, D.K.; Ho, K.; Horn, R.; Silver, E.L.; O’Donnell, K.; Kahn, C.E., Jr. DICOMweb™: Background and application of the web standard for medical imaging. J. Digit. Imaging 2018, 31, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Trobaugh-Lotrario, A.D.; Meyers, R.L.; Tiao, G.M.; Feusner, J.H. Pediatric liver transplantation for hepatoblastoma. Transl. Gastroenterol. Hepatol. 2016, 1, 44. [Google Scholar] [CrossRef] [Green Version]
- Czauderna, P.; Otte, J.B.; Aronson, D.C.; Gauthier, F.; Mackinlay, G.; Roebuck, D.; Plaschkes, J.; Perilongo, G.; Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur. J. Cancer 2005, 41, 1031–1036. [Google Scholar] [CrossRef]
- Kueht, M.; Thompson, P.; Rana, A.; Cotton, R.; O’Mahony, C.; Goss, J. Effects of an early referral system on liver transplantation for hepatoblastoma at Texas Children’s Hospital. Pediatr. Transplant. 2016, 20, 515–522. [Google Scholar] [CrossRef]
- Kasahara, M.; Sakamoto, S.; Fukuda, A. Pediatric living-donor liver transplantation. Semin. Pediatr. Surg. 2017, 26, 224–232. [Google Scholar] [CrossRef]
- von Schweinitz, D.; Hecker, H.; Harms, D.; Bode, U.; Weinel, P.; Bürger, D.; Erttmann, R.; Mildenberger, H. Complete resection before development of drug resistance is essential for survival from advanced hepatoblastoma—A report from the German Cooperative Pediatric Liver Tumor Study HB-89. J. Pediatr. Surg. 1995, 30, 845–852. [Google Scholar] [CrossRef]
- Warmann, S.W.; Fuchs, J. Drug resistance in hepatoblastoma. Curr. Pharm. Biotechnol. 2007, 8, 93–97. [Google Scholar] [CrossRef]
- Lautz, T.B.; Ben-Ami, T.; Tantemsapya, N.; Gosiengfiao, Y.; Superina, R.A. Successful nontransplant resection of POST-TEXT III and IV hepatoblastoma. Cancer 2011, 117, 1976–1983. [Google Scholar] [CrossRef]
- Meyers, R.L.; Czauderna, P.; Otte, J.B. Surgical treatment of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 800–808. [Google Scholar] [CrossRef]
- Uchida, H.; Sakamoto, S.; Sasaki, K.; Takeda, M.; Hirata, Y.; Fukuda, A.; Hishiki, T.; Irie, R.; Nakazawa, A.; Miyazaki, O.; et al. Surgical treatment strategy for advanced hepatoblastoma: Resection versus transplantation. Pediatr. Blood Cancer 2018, 65, e27383. [Google Scholar] [CrossRef]

| Total Enrollment | 1st Session n = 35 | 2nd Session n = 7 | |||
|---|---|---|---|---|---|
| voluntary | consultation | voluntary | consultation | ||
| standard | 43 | 0 | 6 | 0 | 0 |
| intermediate | 36 | 20 | 2 | 6 | 1 |
| high | 15 | 2 | 5 | 0 | 0 |
| 1st Session n = 35 | 2nd Session n = 7 | ||
|---|---|---|---|
| after 2 courses | 2 | 0 | |
| standard | after 4 courses | 3 | 0 |
| after 5 courses | 1 | 0 | |
| after 2 courses | 20 | 0 | |
| intermediate | after 3 courses | 2 | 1 |
| after 4 courses | 0 | 6 | |
| after block A1 | 2 | 0 | |
| high | after block A2 | 2 | 0 |
| after block A3 | 3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hishiki, T.; Honda, S.; Takama, Y.; Inomata, Y.; Okajima, H.; Hoshino, K.; Suzuki, T.; Souzaki, R.; Wada, M.; Kasahara, M.; et al. Feasibility of Real-Time Central Surgical Review for Patients with Advanced-Stage Hepatoblastoma in the JPLT3 Trial. Children 2022, 9, 234. https://doi.org/10.3390/children9020234
Hishiki T, Honda S, Takama Y, Inomata Y, Okajima H, Hoshino K, Suzuki T, Souzaki R, Wada M, Kasahara M, et al. Feasibility of Real-Time Central Surgical Review for Patients with Advanced-Stage Hepatoblastoma in the JPLT3 Trial. Children. 2022; 9(2):234. https://doi.org/10.3390/children9020234
Chicago/Turabian StyleHishiki, Tomoro, Shohei Honda, Yuichi Takama, Yukihiro Inomata, Hideaki Okajima, Ken Hoshino, Tatsuya Suzuki, Ryota Souzaki, Motoshi Wada, Mureo Kasahara, and et al. 2022. "Feasibility of Real-Time Central Surgical Review for Patients with Advanced-Stage Hepatoblastoma in the JPLT3 Trial" Children 9, no. 2: 234. https://doi.org/10.3390/children9020234
APA StyleHishiki, T., Honda, S., Takama, Y., Inomata, Y., Okajima, H., Hoshino, K., Suzuki, T., Souzaki, R., Wada, M., Kasahara, M., Mizuta, K., Oue, T., Yokoi, A., Kazama, T., Komatsu, S., Saeki, I., Miyazaki, O., Takimoto, T., Ida, K., ... Hiyama, E. (2022). Feasibility of Real-Time Central Surgical Review for Patients with Advanced-Stage Hepatoblastoma in the JPLT3 Trial. Children, 9(2), 234. https://doi.org/10.3390/children9020234







