Post-Discharge Effects and Parents’ Opinions of Intranasal Fentanyl with Oral Midazolam Sedation in Pediatric Dental Patients: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Study Protocol
2.2. Questionnaire
2.3. Validity and Reliability of the Questionnaire
2.4. Sample Size
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Post-Sedation Phone-Call Verbal Questionnaire
- Did the patient vomit? (circle one)Yes NoIf yes, how much?.............................................................................................When?...............................................................................................................How soon after sedation?<1 h 2–4 h 4–6 h <6 h
- Did the patient eat? (circle one)Yes NoHow soon after sedation did your child eat?<1 h 2–4 h 4–6 h <6 h
- How long did it take for your child to function normally? (circle one)<1 h 2–4 h 4–6 h <6 h
- Were there any other side effects or complications?……….………………………………………………………………………………
- How would you rate your overall satisfaction with treatment using this medicine? (circle one)
- (a)
- Very unsatisfied
- (b)
- Unsatisfied
- (c)
- Neutral
- (d)
- Satisfied
- (e)
- Very satisfied
- Which behavior best describes your child in the afternoon/evening following the sedation appointment?
- (a)
- Normal
- (b)
- More relaxed than usual
- (c)
- More agitated/aggressive than usual
- (d)
- Very agitated/aggressive
- Please describe your child’s sleep after the sedation appointment.
- (a)
- Normal
- (b)
- Slept more than usual
- (c)
- Awake more than usual
- (d)
- More nightmares than usual
- How many hours after the appointment before the child had normal balance and was able to walk normally? (circle one)<1 h 2–4 h 4–6 h <6 h
- How many hours after the appointment did your child experience normal vision? (no double or blurred vision) (circle one)<1 h 2–4 h 4–6 h <6 hTo be asked after the second sedation visit only:Did you prefer the medication given at the first appointment, second appointment, or no preference?First visit Second visit Same/no preference
References
- Erfanparast, L.; Vafaei, A.; Ranjkesh, B.; Sohrabi, A.; Bahadori, Z.; Pourkazemi, M.; Dadashi, S.; Shirazi, S. Impact of Self-Concept on Preschoolers’ Dental Anxiety and Behavior. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015, 9, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Swarna, K.; Prathima, G.; Suganya, M.; Sanguida, A.; Selvabalaji, A. Recent Advances in Non-Pharmacological Behaviour Management Techniques in Children—An Overview. IOSR J. Dent. Med. Sci. 2019, 18, 18–21. [Google Scholar]
- Lourenço-Matharu, L.; Ashley, P.F.; Furness, S. Sedation of Children Undergoing Dental Treatment. Cochrane Database Syst. Rev. 2012, 12, CD003877. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Reference Manual. Guidelines for Monitoring and Management of Pediatric Patients Before, During, and After Sedation for Diagnostic and Therapeutic Procedures: Update 2019. Pediatric Dent. 2019, 41, 26–52. [Google Scholar]
- American Dental Association Guidelines for the Use of Sedation and General Anesthesia by Dentists. Available online: https://www.ada.org/-/media/project/ada-organization/ada/ada-org/ada/ada/education-and-careers/files/ada_sedation_use_guidelines.pdf?rev=3dea7b89a7fb4a3a98eae26a76af493e&hash=A3A9CABCCDA5BB1D83E88F9146BD8997 (accessed on 31 October 2016).
- Shapira, J.; Kupietzky, A.; Kadari, A.; Fuks, A.B.; Holan, G. Comparison of Oral Midazolam with and Without Hydroxyzine in the Sedation of Pediatric Dental Patients. Pediatr. Dent. 2005, 26, 492–496. [Google Scholar]
- Torres-Pérez, J.; Tapia-García, I.; Rosales-Berber, M.Á.; Hernández-Sierra, J.F.; Pozos-Guillen, A. Comparison of Three Conscious Sedation Regimens for Pediatric Dental Patients. J. Clin. Pediatr. Dent. 2007, 31, 183–186. [Google Scholar] [CrossRef]
- Ahmadi, R.; Mozafar, S.; Bargrizan, M.; Golpayegani, M.V.; Shayeghi, S. Comparison of Nitrous oxide/midazolam and Nitrous oxide/Promethazine for Pediatric Dental Sedation: A Randomized, Cross-Over, Clinical Trial. Dent. Res. J. 2018, 15, 411–419. [Google Scholar] [CrossRef]
- Johnson, E.; Briskie, D.; Majewski, R.; Edwards, S.; Reynolds, P. The Physiologic and Behavioral Effects of Oral and Intranasal Midazolam in Pediatric Dental Patients. Pediatr. Dent. 2010, 32, 229–238. [Google Scholar]
- Tavassoli-Hojjati, S.; Mehran, M.; Haghgoo, R.; Tohid-Rahbari, M.; Ahmadi, R. Comparison of Oral and Buccal Midazolam for Pediatric Dental Sedation: A Randomized, Cross-Over, Clinical Trial for Efficacy, Acceptance and Safety. Iran. J. Pediatr. 2014, 24, 198–206. [Google Scholar]
- Gentz, R.; Casamassimo, P.; Amini, H.; Claman, D.; Smiley, M. Safety and Efficacy of 3 Pediatric Midazolam Moderate Sedation Regimens. Anesth. Prog. 2017, 64, 66–72. [Google Scholar] [CrossRef]
- Papineni, A.; Lourenço-Matharu, L.; Ashley, P. Safety of Oral Midazolam Sedation Use in Paediatric Dentistry: A Review. Int. J. Paediatr. Dent. 2012, 24, 2–13. [Google Scholar] [CrossRef]
- Kılıç, E.T.; Akcay, M.E.; Akdemir, M.S. The Comparison of the Efficacy and Safety of Midazolam, Ketamine, and Midazolam Combined with Ketamine Administered Nasally for Premedication in Children. Anesth. Essays Res. 2018, 12, 489–494. [Google Scholar] [CrossRef]
- Payne, K.; Mattheyse, F.J.; Liebenberg, D.; Dawes, T. The Pharmacokinetics of Midazolam in Paediatric Patients. Eur. J. Clin. Pharmacol. 1989, 37, 267–272. [Google Scholar] [CrossRef]
- Reed, M.D.; Rodarte, A.; Blumer, J.L.; Khoo, K.-C.; Akbari, B.; Pou, S.; Kearns, G.L. The Single-Dose Pharmacokinetics of Midazolam and Its Primary Metabolite in Pediatric Patients After Oral and Intravenous Administration. J. Clin. Pharmacol. 2001, 41, 1359–1369. [Google Scholar] [CrossRef]
- Sado-Filho, J.; Viana, K.A.; Corrêa-Faria, P.; Costa, L.R.; Costa, P.S. Randomized Clinical Trial on the Efficacy of Intranasal or Oral Ketamine-Midazolam Combinations Compared to Oral Midazolam for Outpatient Pediatric Sedation. PLoS ONE 2019, 14, e0213074. [Google Scholar] [CrossRef] [PubMed]
- Manso, M.A.; Guittet, C.; Vandenhende, F.; Granier, L. Efficacy of Oral Midazolam for Minimal and Moderate Sedation in Pediatric Patients: A Systematic Review. Pediatr. Anesth. 2019, 29, 1094–1106. [Google Scholar] [CrossRef]
- Ryan, P.M.; Kienstra, A.J.; Cosgrove, P.; Vezzetti, R.; Wilkinson, M. Safety and Effectiveness of Intranasal Midazolam and Fentanyl Used in Combination in the Pediatric Emergency Department. Am. J. Emerg. Med. 2019, 37, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.P.; Hughes, M.; Mccoy, S.; Crispino, G.; Wakai, A.; O’Sullivan, R. Intranasal Fentanyl for the Prehospital Man-Agement of Acute Pain in Children. Eur. J. Emerg. Med. 2017, 24, 450–454. [Google Scholar] [CrossRef]
- Nemeth, M.; Jacobsen, N.; Bantel, C.; Fieler, M.; Sümpelmann, R.; Eich, C. Intranasal Analgesia and Sedation in Pediatric Emergency Care-A Prospective Observational Study on the Implementation of an Institutional Protocol in a Tertiary Children’s Hospital. Pediatr. Emerg. Care. 2019, 35, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pansini, V.; Curatola, A.; Gatto, A.; Lazzareschi, I.; Ruggiero, A.; Chiaretti, A. Intranasal Drugs for Analgesia and Sedation in Children Admitted to Pediatric Emergency Department: A Narrative Review. Ann. Transl. Med. 2021, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Streisand, J.B.; Zhang, J.; Niu, S.; McJames, S.; Natte, R.; Pace, N.L. MD Buccal Absorption of Fentanyl Is PH-Dependent in Dogs. Anesthesiol. J. Am. Soc. Anesthesiol. 1995, 82, 759–764. [Google Scholar]
- Mamula, P.; Markowitz, J.E.; Neiswender, K.; Zimmerman, A.; Wood, S.; Garofolo, M.; Nieberle, M.; Trautwein, A.; Lombardi, S.; Sargent-Harkins, L.; et al. Safety of Intravenous Midazolam and Fentanyl for Pediatric GI Endoscopy: Prospective Study of 1578 Endoscopies. Gastrointest. Endosc. 2007, 65, 203–210. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.D.; Choi, Y.H.; Han, Y.J.; Suh, D.I. Efficacy and Safety of Fentanyl in Combination with Midazolam in Children on Mechanical Ventilation. J. Korean Med Sci. 2019, 34, 21. [Google Scholar] [CrossRef]
- Williams, M.R.; Nayshtut, M.; Hoefnagel, A.; McKeown, A.; Carlson, D.W.; Cravero, J.; Lightdale, J.; Mason, K.P.; Wilson, S.; Turk, D.C.; et al. Efficacy Outcome Measures for Pediatric Procedural Sedation Clinical Trials. Anesth. Analg. 2018, 126, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.J.; Diekema, D.S.; Paris, C.A.; Quan, L.; Cohen, M.; Seidel, K.D. A Randomized, Clinical Trial of Oral Midazolam Plus Placebo Versus Oral Midazolam Plus Oral Transmucosal Fentanyl for Sedation During Laceration Repair. Pediatrics 2002, 109, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Padmanabhan, M.Y.; Saksena, A.K.; Chandra, G. Midazolam-Fentanyl Analgo-Sedation in Pediatric Dental Patients—A Pilot Study. J. Clin. Pediatr. Dent. 2010, 35, 105–110. [Google Scholar] [CrossRef]
- Oh, S.; Kingsley, K. Efficacy of Ketamine in Pediatric Sedation Dentistry: A Systematic Review. Compend. Contin. Educ. Dent. 2018, 39, e1–e4. [Google Scholar] [PubMed]
- Mohite, V.; Baliga, S.; Thosar, N.; Rathi, N. Role of Dexmedetomidine in Pediatric Dental Sedation. J. Dent. Anesthesia Pain Med. 2019, 19, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Available online: https://pubs.asahq.org/anesthesiology/article/128/3/437/18818/Practice-Guidelines-for-Moderate-Procedural (accessed on 15 March 2018).
- Coteé, C.J.; Karl, H.W.; Notterman, D.A.; Weinberg, J.A.; McCloskey, C. Adverse Sedation Events in Pediatrics: Analysis of Medications Used for Sedation. Pediatrics 2000, 106, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Voepel-Lewis, T.; Prochaska, G.; Tait, A.R. Prolonged Recovery and Delayed Side Effects of Sedation for Diagnostic Imaging Studies in Children. Pediatrics 2000, 105, e42. [Google Scholar] [CrossRef]
- Martinez, D.; Wilson, S. Children Sedated for Dental Care: A Pilot Study of the 24-Hour Postsedation Period. Pediatrics Dent. 2006, 28, 260–264. [Google Scholar]
- Costa, L.R.; Costa, P.S.; Brasileiro, S.V.; Bendo, C.B.; Viegas, C.M.; Paiva, S.M. Post-Discharge Adverse Events Following Pediatric Sedation with High Doses of Oral Medication. J. Pediatrics 2012, 160, 807–813. [Google Scholar] [CrossRef]
- Ritwik, P.; Cao, L.T.; Curran, R.; Musselman, R.J. Post-Sedation Events in Children Sedated for Dental Care. Anesth. Prog. 2013, 60, 54–59. [Google Scholar] [CrossRef]
- McCormack, L.; Chen, J.-W.; Trapp, L.; Job, A. A Comparison of Sedation-Related Events for Two Multiagent Oral Sedation Regimens in Pediatric Dental Patients. Pediatrics Dent. 2014, 36, 302–308. [Google Scholar]
- Huang, A.; Tanbonliong, T. Oral Sedation Postdischarge Adverse Events in Pediatric Dental Patients. Anesth. Prog. 2015, 62, 91–99. [Google Scholar] [CrossRef]
- American Society of Anesthesiologist Physical Status Classification System. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed on 15 October 2014).
- Frankl, S.N. Should the Parent Remain with the Child in the Dental operatory? J. Dent. Child 1962, 29, 150–163. [Google Scholar]
- Mallampati, S.R.; Gatt, S.P.; Gugino, L.D.; Sukumar, P.D.; Waraksa, B.; Freiberger, D.; Liu, P.L. A Clinical Sign to Predict Difficult Tracheal Intubation; A Prospective Study. Can. Anaesth. Soc. J. 1985, 32, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, L. Modern Assessment of Tonsils and Adenoids. Pediatrics Clin. North Am. 1989, 36, 1551–1569. [Google Scholar] [CrossRef]
- El-Mouzan, M.I.; Al-Herbish, A.S.; Al-Salloum, A.A.; Qurachi, M.M.; Al-Omar, A.A. Growth Charts for Saudi Children and Adolescents. Saudi Med. J. 2007, 28, 1555–1568. [Google Scholar]
- Sheta, S.A.; AlSarheed, M. Oral Midazolam Premedication for Children Undergoing General Anaesthesia for Dental Care. Int. J. Pediatrics 2009, 2009, 274380. [Google Scholar] [CrossRef]
- MOH Extemporaneous Formulary Pharmaceutical Services Division Ministry of Health Malaysia. Available online: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/moh-extemporaneous-formulary-2011.pdf (accessed on 15 October 2011).
- Nahata, M.; Pai, V.; Hipple, T. Pediatric Drug Formulation, 6th ed.; Harvey Whitney Books: Cincinnati, OH, USA, 2011. [Google Scholar]
- Rech, M.A.; Barbas, B.; Chaney, W.; Greenhalgh, E.; Turck, C. When to Pick the Nose: Out-of-Hospital and Emergency Department Intranasal Administration of Medications. Ann. Emerg. Med. 2017, 70, 203–211. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry Reference Manual, Preparing for Your Child’s Sedation Visit. Available online: https://www.aapd.org/globalassets/media/policies_guidelines/r_prepostsedation.pdf (accessed on 15 October 2020).
- McQueen, A.; Wright, R.O.; Kido, M.M.; Kaye, E.; Krauss, B. Procedural Sedation and Analgesia Outcomes in Children After Discharge from the Emergency Department: Ketamine Versus fentanyl/midazolam. Ann. Emerg. Med. 2009, 54, 191–197. [Google Scholar] [CrossRef]
- Kennedy, R.M.; Porter, F.L.; Miller, J.P.; Jaffe, D.M. Comparison of Fentanyl/Midazolam with Ketamine/Midazolam for Pediatric Orthopedic Emergencies. Pediatrics 1998, 102, 956–963. [Google Scholar] [CrossRef]
- Hagan, P.P.; Hagan, J.P.; Fields, H.W.; Machen, J.B. The Legal Status of Informed Consent for Behavior Management Techniques in Pediatric Dentistry. Pediatrics Dent. 1984, 6, 204–208. [Google Scholar]
- Kip, G.; Atabek, D.; Bani, M. Comparison of Three Different Ketofol Proportions in Children Undergoing Dental Treatment. Niger. J. Clin. Pr. 2018, 21, 1501–1507. [Google Scholar]
- Lima, A.R.D.A.; Medeiros, M.; Costa, L.R. Mothers’ Perceptions about Pediatric Dental Sedation as an Alternative to Dental General Anesthesia. RGO 2015, 63, 153–160. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, V.B.M.; Costa, L.R.; de Faria, P.C. Parents’ Satisfaction with Paediatric Dental Treatment under Sedation: A cross-sectional Study. Int. J. Paediatr. Dent. 2021, 31, 337–343. [Google Scholar] [CrossRef]
- Lew, V.K.; Lalwani, K.; Palermo, T.M. Factors Affecting Parental Satisfaction Following Pediatric Procedural Sedation. J. Clin. Anesth. 2010, 22, 29–34. [Google Scholar] [CrossRef][Green Version]
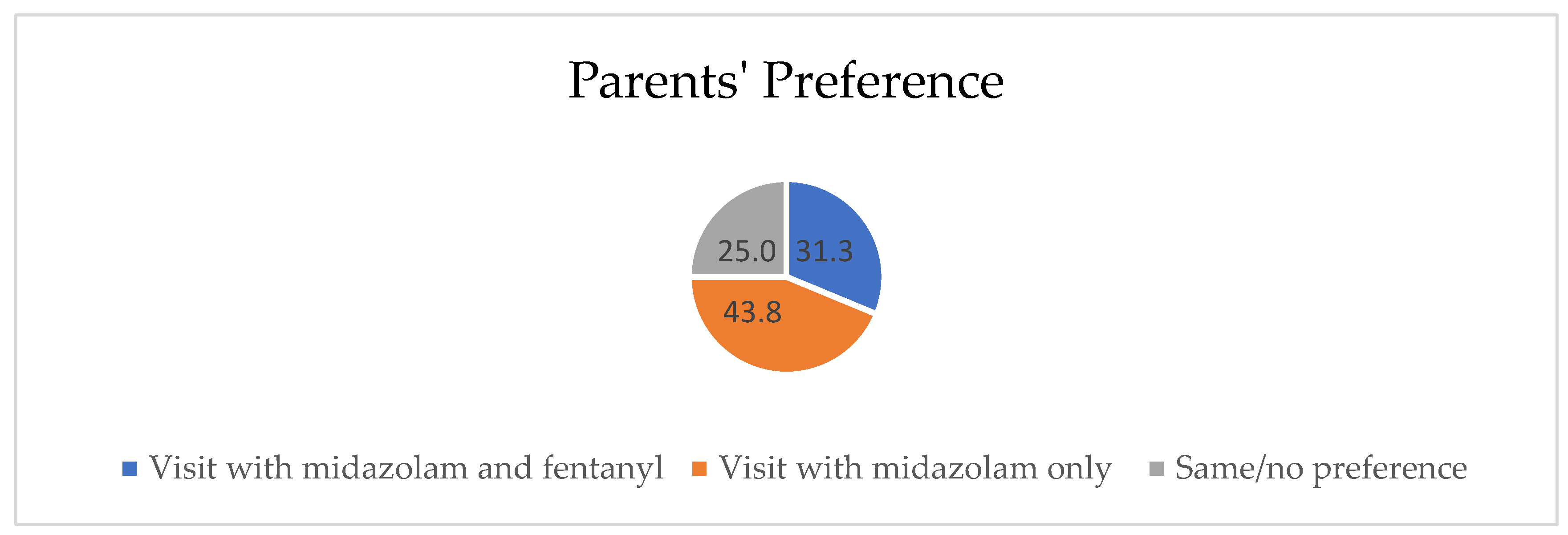
| Number of children | 32 |
| Age (months) | 54.6 ± 10.2 |
| Gender (M/F) | 18/14 |
| Variables | M/F | M | Wilcoxon p-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Vomiting frequency a Children who had a meal a | 2 | 0 | 2 | 0 | 0.564 |
| 1 | 0 | 1 | 0 | 1 | |
| Time until first meal b | 3 | 1 | 2 | 1 | 0.04 * |
| Time until normal function b | 3 | 1 | 3 | 2 | 0.295 |
| Time until normal balance b | 3 | 1 | 3 | 2 | 0.32 |
| Behavior status c | 2 | 2 | 1.5 | 2 | 0.511 |
| Sleeping pattern d | 1 | 1 | 1 | 1 | 0.755 |
| Occurrence of side effects a | 2 | 0 | 2 | 0 | 1 |
| Parent satisfaction e | 4 | 1.75 | 4 | 2 | 0.471 |
| Time | M/F No. of Children (%) | M No. of Children (%) |
|---|---|---|
| <1 h | 0 (0%) | 1 (3.2%) |
| 2–4 h | 13 (41.9%) | 19 (61.3%) |
| 4–6 h | 11 (35.5%) | 9 (29%) |
| >6 h | 7 (22.6%) | 2 (6.5%) |
| Total | 32 (100%) | 32 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaidari, R.I.; AlSarheed, M.A. Post-Discharge Effects and Parents’ Opinions of Intranasal Fentanyl with Oral Midazolam Sedation in Pediatric Dental Patients: A Cross-Sectional Study. Children 2022, 9, 142. https://doi.org/10.3390/children9020142
Alhaidari RI, AlSarheed MA. Post-Discharge Effects and Parents’ Opinions of Intranasal Fentanyl with Oral Midazolam Sedation in Pediatric Dental Patients: A Cross-Sectional Study. Children. 2022; 9(2):142. https://doi.org/10.3390/children9020142
Chicago/Turabian StyleAlhaidari, Roaa I., and Maha A. AlSarheed. 2022. "Post-Discharge Effects and Parents’ Opinions of Intranasal Fentanyl with Oral Midazolam Sedation in Pediatric Dental Patients: A Cross-Sectional Study" Children 9, no. 2: 142. https://doi.org/10.3390/children9020142
APA StyleAlhaidari, R. I., & AlSarheed, M. A. (2022). Post-Discharge Effects and Parents’ Opinions of Intranasal Fentanyl with Oral Midazolam Sedation in Pediatric Dental Patients: A Cross-Sectional Study. Children, 9(2), 142. https://doi.org/10.3390/children9020142





