Diagnostic Efficacy of Rectal Suction Biopsy with Regard to Weight in Children Investigated for Hirschsprung’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data
2.2. Investigation Routines
2.3. Biopsy Technique and Staining
2.4. Statistical Analysis
3. Ethical Considerations
4. Results
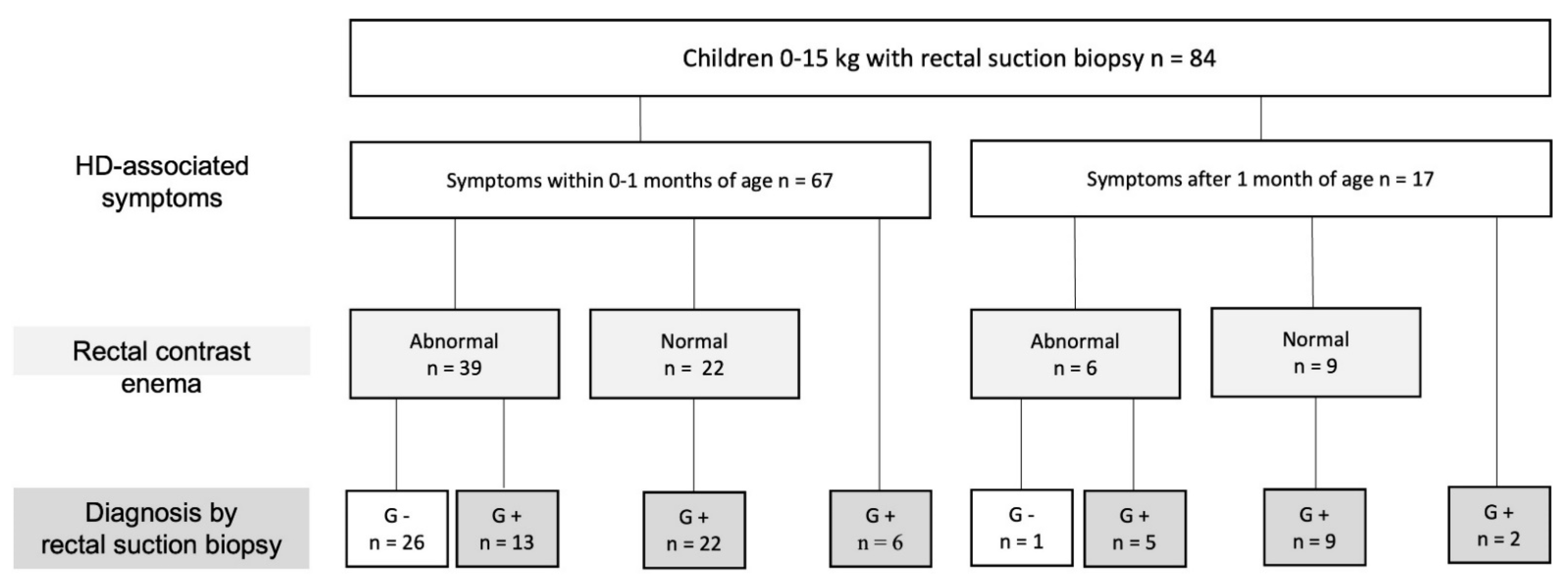
4.1. Patients
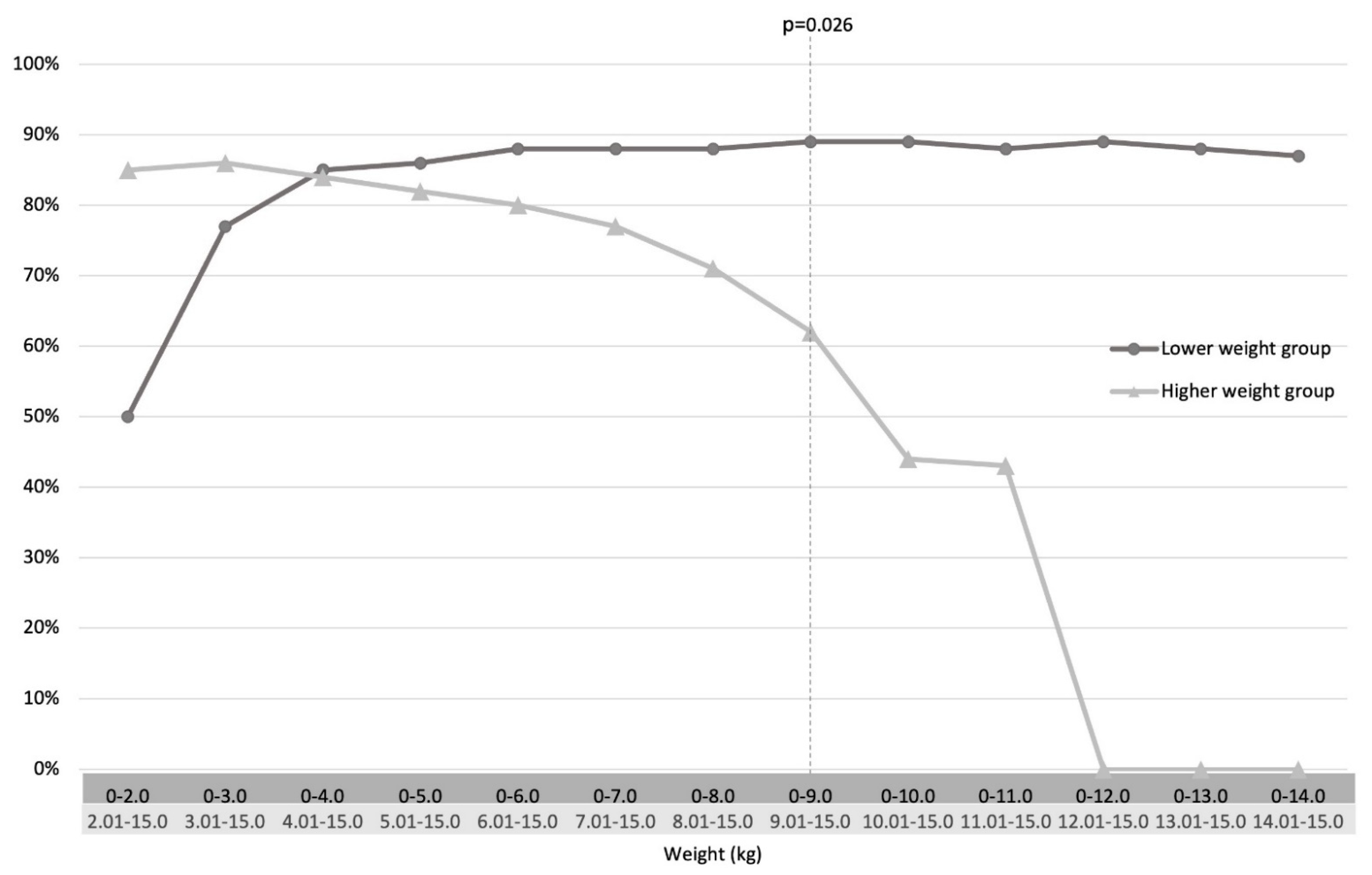
4.2. Rectal Suction Biopsy (RSB) Data and Efficacy
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HD | Hirschsprung disease |
| RSB | rectal suction biopsy |
| FTB | full thickness biopsy |
References
- Heanue, T.A.; Pachnis, V. Enteric nervous system development and Hirschsprung’s disease: Advances in genetic and stem cell studies. Nat. Rev. Neurosci. 2007, 8, 466–479. [Google Scholar] [CrossRef]
- Friedmacher, F.; Puri, P. Current practice patterns of rectal suction biopsy in the diagnostic work-up of Hirschsprung’s disease: Results from an international survey. Pediatr. Surg. Int. 2016, 32, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, W.O., 3rd; Bill, A.H., Jr. Diagnosis of Hirschsprung’s Disease Excluded by Rectal Suction Biopsy. N. Engl. J. Med. 1965, 272, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Martucciello, G.; Prato, A.P.; Puri, P.; Holschneider, A.M.; Meier-Ruge, W.; Jasonni, V.; Tovar, J.A.; Grosfeld, J.L. Controversies concerning diagnostic guidelines for anomalies of the enteric nervous system: A report from the fourth International Symposium on Hirschsprung’s disease and related neurocristopathies. J. Pediatr. Surg. 2005, 40, 1527–1531. [Google Scholar] [CrossRef]
- Friedmacher, F.; Puri, P. Rectal suction biopsy for the diagnosis of Hirschsprung’s disease: A systematic review of diagnostic accuracy and complications. Pediatr. Surg. Int. 2015, 31, 821–830. [Google Scholar] [CrossRef]
- Brady, A.C.; Saito, J.M.; Lukas, K.; Guthrie, T.; Utterson, E.C.; White, F.V.; Dillon, P.A. Suction rectal biopsy yields adequate tissue in children. J. Pediatr. Surg. 2016, 51, 966–969. [Google Scholar] [CrossRef]
- Croffie, J.M.; Davis, M.M.; Faught, P.R.; Corkins, M.R.; Gupta, S.K.; Pfefferkorn, M.D.; Molleston, J.P.; Fitzgerald, J.F. At what age is a suction rectal biopsy less likely to provide adequate tissue for identification of ganglion cells? J. Pediatr. Gastroenterol. Nutr. 2007, 44, 198–202. [Google Scholar] [CrossRef]
- Pini-Prato, A.; Martucciello, G.; Jasonni, V. Rectal suction biopsy in the diagnosis of intestinal dysganglionoses: 5-year experience with Solo-RBT in 389 patients. J. Pediatr. Surg. 2006, 41, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Hing, C.I.N.L.; Teng, R.; Porrett, L.; Thompson, R. Comparison of inconclusive rates between suction rectal biopsy and open strip rectal biopsy in children of different age groups: A single-center retrospective study. World J. Pediatr. Surg. 2020, 3, e00080. [Google Scholar]
- Hall, N.J.; Kufeji, D.; Keshtgar, A. Out with the old and in with the new: A comparison of rectal suction biopsies with traditional and modern biopsy forceps. J. Pediatr. Surg. 2009, 44, 395–398. [Google Scholar] [CrossRef]
- Kapur, R.P. Practical pathology and genetics of Hirschsprung’s disease. Semin. Pediatr. Surg. 2009, 18, 212–223. [Google Scholar] [CrossRef]
- Khan, A.R.; Vujanic, G.M.; Huddart, S. The constipated child: How likely is Hirschsprung’s disease? Pediatr. Surg. Int. 2003, 19, 439–442. [Google Scholar] [CrossRef]
- Alizai, N.K.; Batcup, G.; Dixon, M.F.; Stringer, M.D. Rectal biopsy for Hirschsprung’s disease: What is the optimum method? Pediatr. Surg. Int. 1998, 13, 121–124. [Google Scholar] [CrossRef]
- Muise, E.D.; Hardee, S.; Morotti, R.A.; Cowles, R.A. A comparison of suction and full-thickness rectal biopsy in children. J. Surg. Res. 2016, 201, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Meinds, R.J.; Kuiper, G.A.; Parry, K.; Timmer, A.; Groen, H.; Heineman, E.; Broens, P.M. Infant’s Age Influences the Accuracy of Rectal Suction Biopsies for Diagnosing of Hirschsprung’s Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- von Steyern, K.V.; Wingren, P.; Wiklund, M.; Stenström, P.; Arnbjörnsson, E. Visualisation of the rectoanal inhibitory reflex with a modified contrast enema in children with suspected Hirschsprung disease. Pediatr. Radiol. 2013, 43, 950–957. [Google Scholar] [CrossRef][Green Version]
- Ax, S.Ö.; Arnbjörnsson, E.; Nord, D.G. A Comparison of Rectal Suction and Full Wall Biopsy in Hirschsprung’s Disease. Surg. Sci. 2014, 5, 15–19. [Google Scholar] [CrossRef][Green Version]
- Allen, A.R.; Putnam, A.R.; Presson, A.P.; Allen, C.M.; Barnhart, D.C.; Rollins, M.D. Accuracy of Suction Rectal Biopsy for Diagnosis of Hirschsprung’s Disease in Neonates. Eur. J. Pediatr. Surg. 2019, 29, 425–430. [Google Scholar] [CrossRef]
- Halleran, D.R.; Ahmad, H.; Lehmkuhl, H.; Baker, P.; Wood, R.J.; Levitt, M.A.; Fisher, J.G. Suction Rectal Biopsy is Accurate in Late Preterm Infants with Suspected Hirschsprung Disease. J. Pediatr. Surg. 2020, 55, 67–70. [Google Scholar] [CrossRef] [PubMed]
- de Lorijn, F.; Kremer, L.C.; Reitsma, J.B.; Benninga, M.A. Diagnostic tests in Hirschsprung disease: A systematic review. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 496–505. [Google Scholar] [CrossRef] [PubMed]
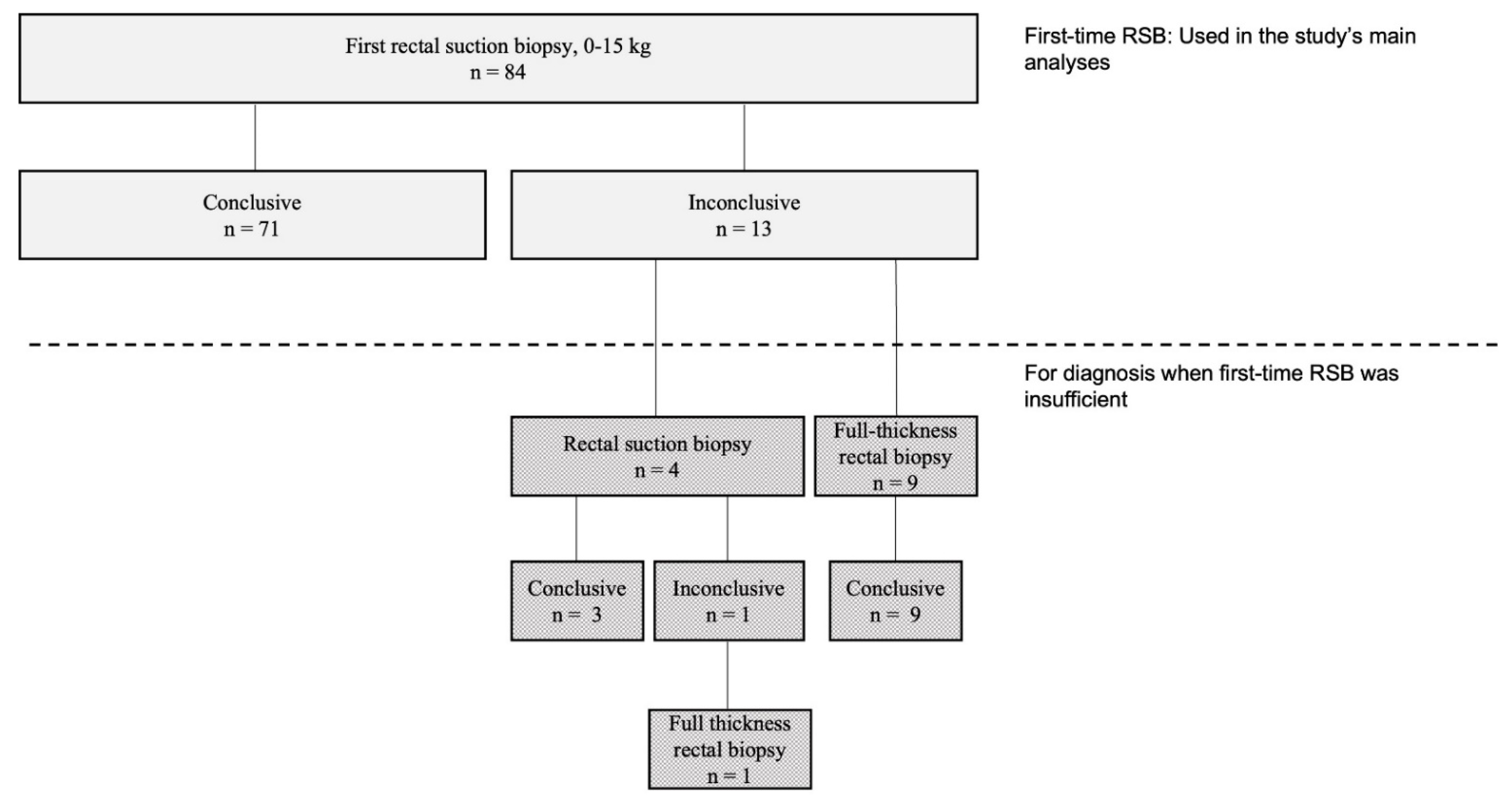

| All First Time RSB n = 84 | First-Time RSB with Conclusive Diagnostics n = 71 | First-Time RSB with Inconclusive Diagnostics n = 13 | p-Value | |
|---|---|---|---|---|
| Gender (female) | 29 (35) | 26 (37) | 3 (23) | |
| Age at biopsy (days) | 42 (1–1345) | 58 (1–860) | 26 (2–1345) | 0.508 a |
| Weight at biopsy (kg) | 4.0 (1.6–14.8) | 4.0 (1.6–12.0) | 3.8 (1.8–14.8) | 0.504 a |
| Diagnostic efficacy at first biopsy | 71 (85) | |||
| Diagnosed with HD (n = 27) | 27 (32) | 20 (28) | 7 (54) | 0.104 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fransson, E.; Granéli, C.; Hagelsteen, K.; Tofft, L.; Hambraeus, M.; Munoz Mitev, R.U.; Gisselsson, D.; Stenström, P. Diagnostic Efficacy of Rectal Suction Biopsy with Regard to Weight in Children Investigated for Hirschsprung’s Disease. Children 2022, 9, 124. https://doi.org/10.3390/children9020124
Fransson E, Granéli C, Hagelsteen K, Tofft L, Hambraeus M, Munoz Mitev RU, Gisselsson D, Stenström P. Diagnostic Efficacy of Rectal Suction Biopsy with Regard to Weight in Children Investigated for Hirschsprung’s Disease. Children. 2022; 9(2):124. https://doi.org/10.3390/children9020124
Chicago/Turabian StyleFransson, Emma, Christina Granéli, Kristine Hagelsteen, Louise Tofft, Mette Hambraeus, Rodrigo Urdar Munoz Mitev, David Gisselsson, and Pernilla Stenström. 2022. "Diagnostic Efficacy of Rectal Suction Biopsy with Regard to Weight in Children Investigated for Hirschsprung’s Disease" Children 9, no. 2: 124. https://doi.org/10.3390/children9020124
APA StyleFransson, E., Granéli, C., Hagelsteen, K., Tofft, L., Hambraeus, M., Munoz Mitev, R. U., Gisselsson, D., & Stenström, P. (2022). Diagnostic Efficacy of Rectal Suction Biopsy with Regard to Weight in Children Investigated for Hirschsprung’s Disease. Children, 9(2), 124. https://doi.org/10.3390/children9020124






