Giant Bilateral Hydronephrosis in A Newborn—A Case Report
Abstract
1. Introduction
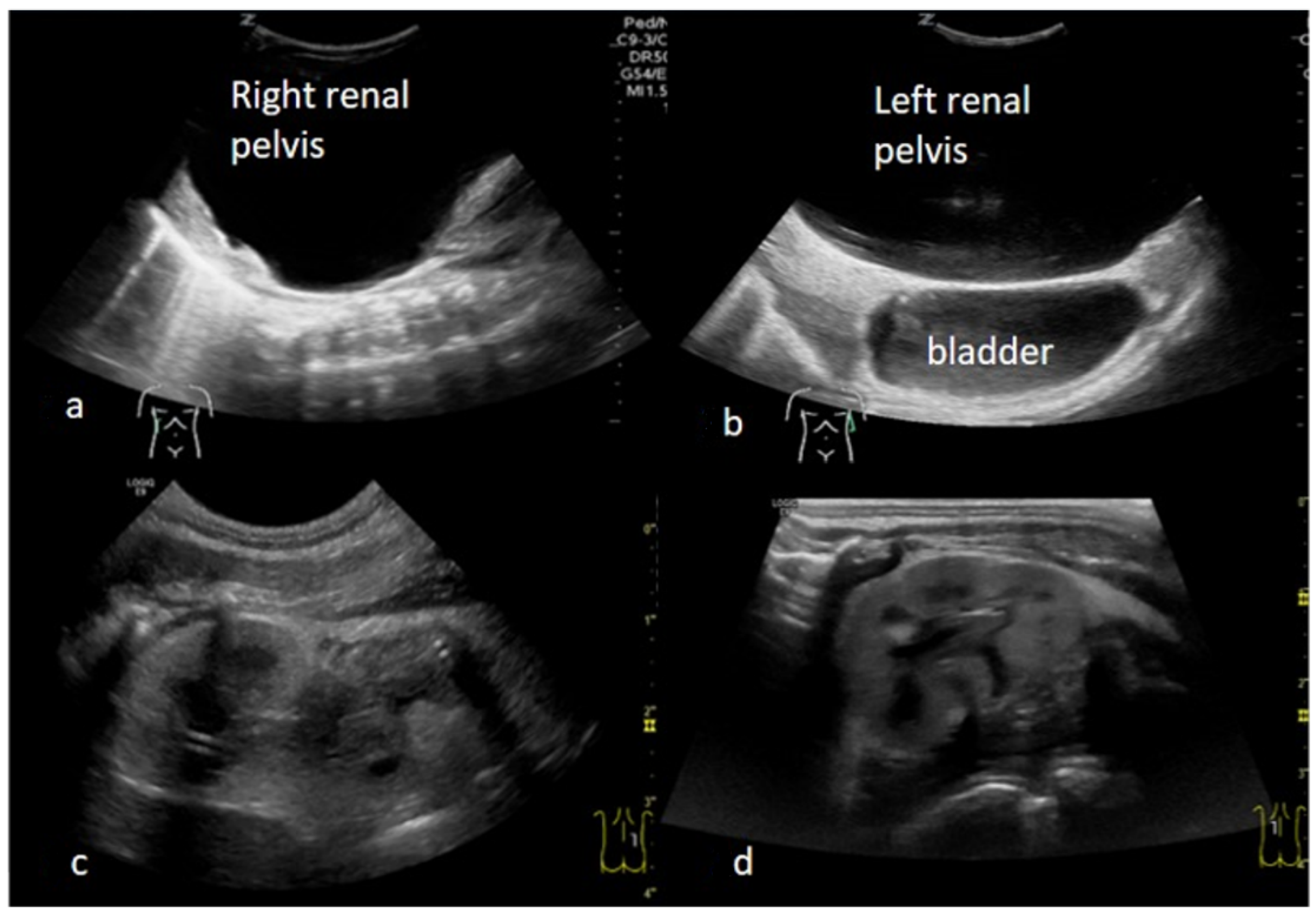
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarhan, O.M.; El Helaly, A.; Al Otay, A.H.; Al Ghanbar, M.; Nakshabandi, Z.N. Prenatally detected, unilateral, high-grade hydronephrosis: Can we predict the natural history? Can. Urol. Assoc. J. 2018, 12, E137–E141. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Pandit, A.; Roy, S.; Sahoo, S.K. Congenital giant hydronephrosis: A rare cause for upper abdominal mass in the newborn. J. Clin. Neonatol. 2013, 2, 33–35. [Google Scholar] [CrossRef]
- Benhaddou, H.; Bellahcen, M.; Elazzouzi, D. Hydronéphrose géante chez l’enfant: À propos d’une observation [Giant hydronephrosis in a child: Case report]. Arch. Pediatr. 2013, 20, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Kaura, K.S.; Kumar, M.; Sokhal, A.K.; Gupta, A.K.; Purkait, B.; Saini, D.; Sankhwar, S. Giant hydronephrosis: Still a reality! Turk. J. Urol. 2017, 43, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Benchekroun, A.; Alami, M.; Ghadouane, M.; Zanoud, M.; Nouini, Y.; Benslimane, L.; Belahnech, Z.; Faik, M. Hydronéphrose géante: À propos de deux cas. Ann. Urol. 2003, 37, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Uson, A.C.; Levitt, S.B.; Lattimer, J.K. Giant hydronephrosis in children. Pediatrics 1969, 44, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Crooks, K.K.; Hendren, W.H.; Pfister, R.C. Giant hydronephrosis in children. J. Pediatr. Surg. 1979, 14, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Matsumoto, F.; Kawagoe, M.; Matsui, F. Urological emergency in neonates with congenital hydronephrosis. Int. J. Urol. 2007, 14, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, I.; Bahouth, Z.; Halachmi, S. Giant Congenital Hydronephrosis Obstructing the Gastro Intestinal System and the Contralateral Kidney in a New Born. Urol. Case Rep. 2016, 8, 1–3. [Google Scholar] [CrossRef][Green Version]
- Nerli, R.; Reddy, M.; Hiremath, M.; Shishir, D.; Patil, S.; Guntaka, A. Surgical outcomes of laparoscopic dismembered pyeloplasty in children with giant hydronephrosis secondary to ureteropelvic junction obstruction. J. Pediatr. Urol. 2012, 8, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Park, K.; Choi, H. Long-term Outcomes of Dismembered Pyeloplasty for Midline-crossing Giant Hydronephrosis Caused by Ureteropelvic Junction Obstruction in Children. Urology 2010, 76, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Has, R.; Sivrikoz, T.S. Prenatal Diagnosis and Findings in Ureteropelvic Junction Type Hydronephrosis. Front. Pediatr. 2020, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Liu, C.; Li, X.; Sun, H. Diagnostic Value of Anteroposterior Diameter of Renal Pelvis for Predicting Postnatal Surgery: A Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.; Meyers, M.; Brodie, K.; Palmer, C.; Campbell, J. Inter-rater reliability of the APD, SFU and UTD grading systems in fetal sonography and MRI. J. Pediatr. Urol. 2016, 12, 305.e1–305.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.B.; Armstrong, W.R.; Maizels, M. Hydronephrosis: Prenatal and postnatal evaluation and management. Clin. Perinatol. 2014, 41, 661–678. [Google Scholar] [CrossRef]
- Lence, T.; Lockwood, G.M.; Storm, D.W.; Ward, C.E.; Cooper, C.S. The utility of renal sonographic measurements in differentiating children with high grade congenital hydronephrosis. J. Pediatr. Urol. 2021, 17, 660.e1–660.e9. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Singh, K.; Sun, R.C.; Nassr, A.A. Evaluation and fetal intervention in severe fetal hydronephrosis. Curr. Opin. Pediatr. 2021, 33, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Župančić, B.; Popović, L.; Mikulić, D.; Vrtar, Z.; Fattorini, I.; Augustin, G. Calyceal Plication with Pyeloplasty in the Treatment of Giant Hydronephrosis in Children. Eur. J. Pediatr. Surg. 2006, 16, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Lopez, C.; Baud, C.; Veyrac, C.; Morin, D.; Averous, M. Hydronéphrose géante du nouveau-né: Résultats a long-terme de la néphroplicature primaire néonatale [Congenital giant hydronephrosis: Long-term results of primary neonatal nephroplication]. Prog. Urol. 2006, 16, 481–484. [Google Scholar] [PubMed]
- Kato, Y.; Yamataka, A.; Okazaki, T.; Yanai, T.; Lane, G.J.; Kobayashi, H.; Someya, T.; Yamashiro, Y.; Miyano, T. Surgical treatment and outcome of mega-hydronephrosis due to pelviureteric junction stenosis. Pediatr. Surg. Int. 2006, 22, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Belman, A.B.; Gil Rushton, H. Kidney Folding: The Y-Plasty—A Means of Creating a Dependent Ureteropelvic Junction in the Child With Giant Hydronephrosis. J. Urol. 2007, 178, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Danish, N.; Yadav, P.; Kaushik, V.N.; Kakoti, S.; Kumar, A.; Banthia, R.; Srivastava, A. Role of ureterocalicostomy in management of giant hydronephrosis in children in contemporary practice: Indications, outcomes and challenges. J. Pediatr. Urol. 2021, 17, 657.e1–657.e7. [Google Scholar] [CrossRef] [PubMed]
- Karnak, I.; Woo, L.L.; Shah, S.N.; Sirajuddin, A.; Kay, R.; Ross, J.H. Prenatally detected ureteropelvic junction obstruction: Clinical features and associated urologic abnormalities. Pediatr. Surg. Int. 2008, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, U.S.; Parekh, P.R.; Tullu, M.S. Soto’s Syndrome with bilateral hydronephrosis and hydroureters. Indian J. Pediatr. 2002, 69, 913–915. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frech-Dörfler, M.; Durand, S.; Prüfer, F.; Holland-Cunz, S.; Rudin, C. Giant Bilateral Hydronephrosis in A Newborn—A Case Report. Children 2022, 9, 1890. https://doi.org/10.3390/children9121890
Frech-Dörfler M, Durand S, Prüfer F, Holland-Cunz S, Rudin C. Giant Bilateral Hydronephrosis in A Newborn—A Case Report. Children. 2022; 9(12):1890. https://doi.org/10.3390/children9121890
Chicago/Turabian StyleFrech-Dörfler, Martina, Sabrina Durand, Friederike Prüfer, Stefan Holland-Cunz, and Christoph Rudin. 2022. "Giant Bilateral Hydronephrosis in A Newborn—A Case Report" Children 9, no. 12: 1890. https://doi.org/10.3390/children9121890
APA StyleFrech-Dörfler, M., Durand, S., Prüfer, F., Holland-Cunz, S., & Rudin, C. (2022). Giant Bilateral Hydronephrosis in A Newborn—A Case Report. Children, 9(12), 1890. https://doi.org/10.3390/children9121890





