Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder
Abstract
1. Introduction
2. Physical Activity with a Special Emphasis on ASD
Physical Exercise Interventions for Children and Adolescents with ASD
3. Gut Microbiota in Children and Adolescents with ASD
3.1. Metabolomic Studies in ASD
3.2. Microbiota–Gut–Brain Axis in ASD
3.3. Modulation of Intestinal Microbiota as Treatment for Youth with ASD
4. Genetics of ASD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Institute of Mental Health. Autism Spectrum Disorder. Available online: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd#:~:text=Autism%20spectrum%20disorder%20(ASD)%20is,first%20two%20years%20of%20life (accessed on 5 October 2022).
- Department of Health and Human Services; National Institutes of Health; National Institute of Mental Health. (Updated 2021). NIMH Strategic Plan for Research (NIH Publication No. 20-MH-8120). Available online: https://www.nimh.nih.gov/sites/default/files/documents/about/strategic-planning-reports/NIMH-Strategic-Plan-for-Research-2021-Update.pdf (accessed on 2 September 2022).
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Higgins, J.P.; Brayne, C.E. Systematic review of prevalence studies of autism spectrum disorders. Arch. Dis. Child 2006, 91, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Autism. Available online: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders (accessed on 2 September 2022).
- Morales-Hidalgo, P.; Roigé-Castellví, J.; Hernandez-Martinez, C.; Voltas, N.; Canals, J. Prevalence and characteristics of autism spectrum disorder among Spanish school-age children. J. Autism Dev. Disord. 2018, 48, 3176–3190. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146. [Google Scholar] [CrossRef]
- Frazier, T.W.; Georgiades, S.; Bishop, S.L.; Hardan, A.Y. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 329–340.e323. [Google Scholar] [CrossRef]
- Cherskov, A.; Pohl, A.; Allison, C.; Zhang, H.; Payne, R.A.; Baron-Cohen, S. Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Transl. Psychiatry 2018, 8, 136. [Google Scholar] [CrossRef]
- Ferri, S.L.; Abel, T.; Brodkin, E.S. Sex differences in autism spectrum disorder: A review. Curr. Psychiatry Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Beggiato, A.; Peyre, H.; Maruani, A.; Scheid, I.; Rastam, M.; Amsellem, F.; Gillberg, C.I.; Leboyer, M.; Bourgeron, T.; Gillberg, C. Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res. 2017, 10, 680–689. [Google Scholar] [CrossRef]
- Lai, M.C.; Lerch, J.P.; Floris, D.L.; Ruigrok, A.N.; Pohl, A.; Lombardo, M.V.; Baron-Cohen, S. Imaging sex/gender and autism in the brain: Etiological implications. J. Neurosci. Res. 2017, 95, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Singer, L. Thoughts about sex and gender differences from the next generation of autism scientists. Mol. Autism 2015, 6, 52. [Google Scholar] [CrossRef][Green Version]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, A.B.; Turner, T.N.; Murali, S.C.; Hsieh, P.; Sulovari, A.; Wang, T.; Coe, B.P.; Guo, H.; Hoekzema, K.; Bakken, T.E.; et al. Recent ultra-rare inherited variants implicate new autism candidate risk genes. Nat. Genet. 2021, 53, 1125–1134. [Google Scholar] [CrossRef]
- Buxbaum, J.D. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin. Neurosci. 2022, 11, 35–43. [Google Scholar] [CrossRef]
- Newschaffer, C.J.; Fallin, D.; Lee, N.L. Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiol. Rev. 2002, 24, 137–153. [Google Scholar] [CrossRef]
- Stoner, R.; Chow, M.L.; Boyle, M.P.; Sunkin, S.M.; Mouton, P.R.; Roy, S.; Wynshaw-Boris, A.; Colamarino, S.A.; Lein, E.S.; Courchesne, E. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 2014, 370, 1209–1219. [Google Scholar] [CrossRef]
- Zhang, S. Prenatal Diagnosis of Autism. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Francino, M.P. Birth Mode-Related Differences in Gut Microbiota Colonization and Immune System Development. Ann. Nutr. Metab. 2018, 73 (Suppl. 3), 12–16. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.; Man, W.H.; Chu, M.; Arp, K.; Watson, R.L.; Sanders, E.A.M.; Fuentes, S.; et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef]
- Al-Zalabani, A.H.; Al-Jabree, A.H.; Zeidan, Z.A. Is cesarean section delivery associated with autism spectrum disorder? Neurosci. J. 2019, 24, 11–15. [Google Scholar] [CrossRef]
- Curran, E.A.; O’Neill, S.M.; Cryan, J.F.; Kenny, L.C.; Dinan, T.G.; Khashan, A.S.; Kearney, P.M. Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Child Psychol. Psychiatry 2015, 56, 500–508. [Google Scholar] [CrossRef]
- Curran, E.A.; Cryan, J.F.; Kenny, L.C.; Dinan, T.G.; Kearney, P.M.; Khashan, A.S. Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J. Autism Dev. Disord. 2016, 46, 603–614. [Google Scholar] [CrossRef]
- Gregory, S.G.; Anthopolos, R.; Osgood, C.E.; Grotegut, C.A.; Miranda, M.L. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990–1998) and Education Research (1997–2007) databases. JAMA Pediatr. 2013, 167, 959–966. [Google Scholar] [CrossRef]
- Dodds, L.; Fell, D.B.; Shea, S.; Armson, B.A.; Allen, A.C.; Bryson, S. The role of prenatal, obstetric and neonatal factors in the development of autism. J. Autism Dev. Disord. 2011, 41, 891–902. [Google Scholar] [CrossRef]
- Yip, B.H.K.; Leonard, H.; Stock, S.; Stoltenberg, C.; Francis, R.W.; Gissler, M.; Gross, R.; Schendel, D.; Sandin, S. Caesarean section and risk of autism across gestational age: A multi-national cohort study of 5 million births. Int. J. Epidemiol. 2017, 46, 429–439. [Google Scholar] [CrossRef]
- Chen, H.; Tan, D. Cesarean Section or Natural Childbirth? Cesarean Birth May Damage Your Health. Front. Psychol. 2019, 10, 351. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, J.; Yang, B. Targeting gut microbiome: A novel and potential therapy for autism. Life Sci. 2018, 194, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.A.; MacFabe, D.F.; Kavaliers, M.; Ossenkopp, K.-P. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: Relevance to autism spectrum disorders. Behav. Brain Res. 2015, 278, 244–256. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.; Dinan, T.G.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Hornig, M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr. Opin. Rheumatol. 2013, 25, 488–795. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.-L.; Nelson, M.N.; Wexler, H.M. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Aragon-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Alvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef]
- Ramos, C.; Gibson, G.R.; Walton, G.E.; Magistro, D.; Kinnear, W.; Hunter, K. Systematic review of the effects of exercise and physical activity on the gut microbiome of older adults. Nutrients 2022, 14, 674. [Google Scholar] [CrossRef]
- Mc Gettigan, N.; O’Toole, A.; Boland, K. “Role of exercise in preventing and restoring gut dysbiosis in patients with inflammatory bowel disease”: A letter to the editor. World J. Gastroenterol. 2022, 28, 878. [Google Scholar] [CrossRef]
- Huang, J.; Du, C.; Liu, J.; Tan, G. Meta-Analysis on Intervention Effects of Physical Activities on Children and Adolescents with Autism. Int. J. Environ. Res. Public Health 2020, 17, 1950. [Google Scholar] [CrossRef]
- Ning, N.; Zhang, Y.; Yang, G. Review of studies on repetitive stereotyped behaviors in children with autism spectrum disorders. Spec. Educ. China 2015, 2, 46–52. [Google Scholar]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Participants, S.T.C.P. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sport Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kostyrka-Allchorne, K.; Cooper, N.R.; Simpson, A. The relationship between television exposure and children’s cognition and behaviour: A systematic review. Dev. Rev. 2017, 44, 19–58. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Carson, V.; Chaput, J.P.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-h Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41, S311–S327. [Google Scholar] [CrossRef]
- Hidding, L.M.; Chinapaw, M.J.M.; Belmon, L.S.; Altenburg, T.M. Co-creating a 24-h movement behavior tool together with 9–12-year-old children using mixed-methods: MyDailyMoves. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.P.; Irwin, J.M.; Rosen, A.B.; Baldwin, J.; Schenkelberg, M. Comparison of Physical Activity between Children with and Without Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Adapt. Phys. Act. Q. 2022, 39, 456–481. [Google Scholar] [CrossRef]
- Jackson, S.L.J.; Abel, E.A.; Reimer, S.; McPartland, J.C. Brief Report: A Specialized Fitness Program for Individuals with Autism Spectrum Disorder Benefits Physical, Behavioral, and Emotional Outcomes. J. Autism Dev. Disord. 2022, 1–9. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Malow, B.A. Sleep and autism spectrum disorders. Pediatr. Clin. N. Am. 2011, 58, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, J.; Healy, S.; MacDonald, M.; Geldhof, J.; Palmiere, K.; Haegele, J.A. Physical activity and screen time among youth with autism: A longitudinal analysis from 9 to 18 years. Autism 2021, 25, 1090–1099. [Google Scholar] [CrossRef]
- MacMullin, J.A.; Lunsky, Y.; Weiss, J.A. Plugged in: Electronics use in youth and young adults with autism spectrum disorder. Autism 2016, 20, 45–54. [Google Scholar] [CrossRef]
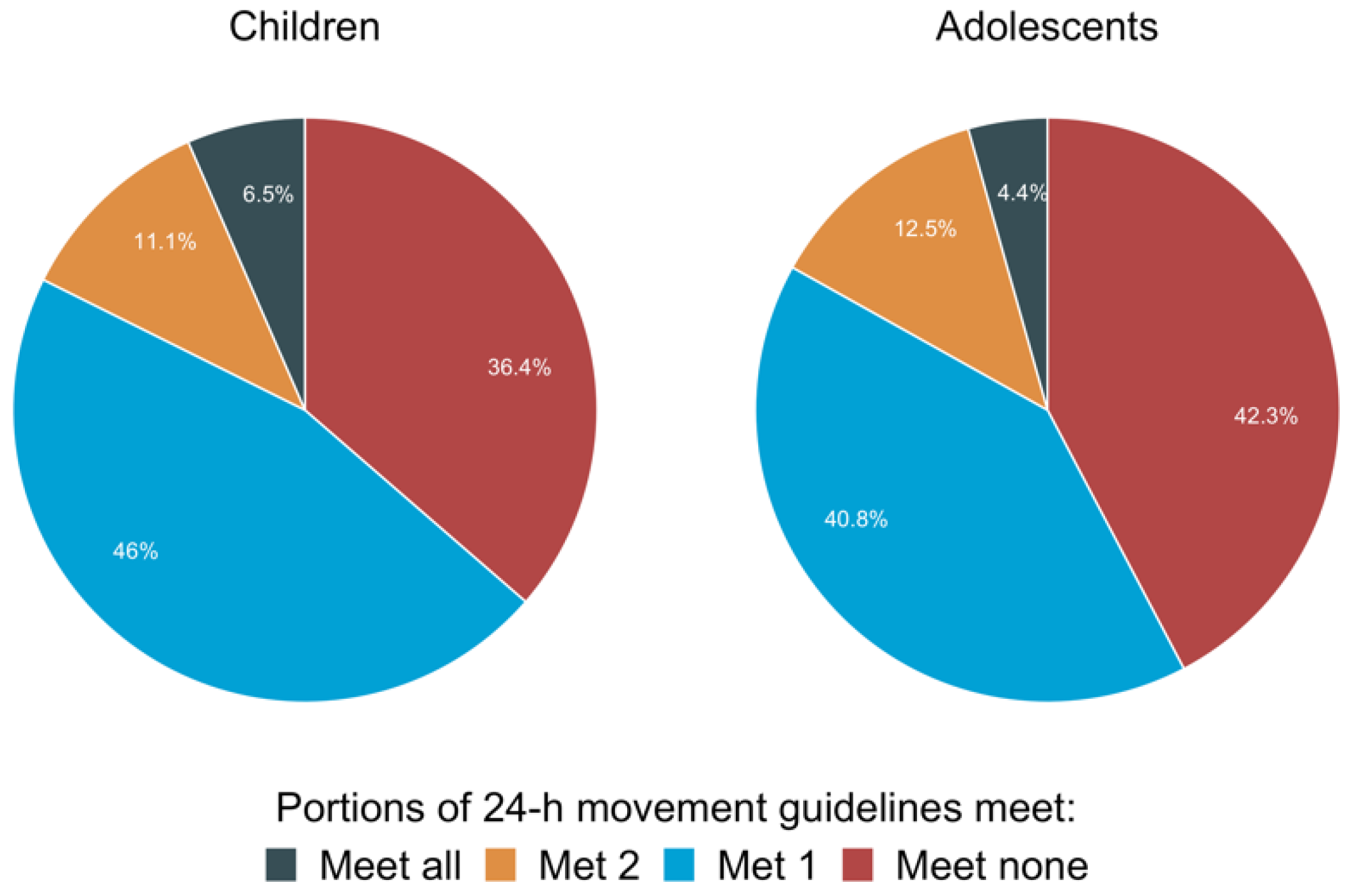
- Healy, S.; Aigner, C.J.; Haegele, J.A.; Patterson, F. Meeting the 24-h movement guidelines: An update on US youth with autism spectrum disorder from the 2016 National Survey of Children’s Health. Autism Res. 2019, 12, 941–951. [Google Scholar] [CrossRef]
- Healy, S.; Foley, J.; Haegele, J.A. Physical Activity, Screen Time, and Sleep Duration among Youth with Chronic Health Conditions in the United States. Am. J. Health Promot. 2020, 34, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Haegele, J.A.; Sun, F.; Alves, M.L.T.; Ang, S.H.C.; Lee, J.; Ng, K.; dos Santos Alves, I.; Healy, S.; Huang, W.Y. Meeting the 24-h movement guidelines and health-related outcomes among youth with autism spectrum disorder: A seven-country observational study. Child Adolesc. Psychiatry Ment. Health 2022, 16, 50. [Google Scholar] [CrossRef]
- Barker, J.; Smith Byrne, K.; Doherty, A.; Foster, C.; Rahimi, K.; Ramakrishnan, R.; Woodward, M.; Dwyer, T. Physical activity of UK adults with chronic disease: Cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK Biobank participants. Int. J. Epidemiol. 2019, 48, 1167–1174. [Google Scholar] [CrossRef]
- Healy, S.; Aigner, C.J.; Haegele, J.A. Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism 2019, 23, 1046–1050. [Google Scholar] [CrossRef]
- Matheson, B.E.; Douglas, J.M. Overweight and obesity in children with autism spectrum disorder (ASD): A critical review investigating the etiology, development, and maintenance of this relationship. Rev. J. Autism Dev. Disord. 2017, 4, 142–156. [Google Scholar] [CrossRef]
- Franke, K.B.; Hills, K.; Huebner, E.S.; Flory, K. Life Satisfaction in Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 1205–1218. [Google Scholar] [CrossRef]
- McCoy, S.M.; Morgan, K. Obesity, physical activity, and sedentary behaviors in adolescents with autism spectrum disorder compared with typically developing peers. Autism 2020, 24, 387–399. [Google Scholar] [CrossRef]
- Leader, G.; Flynn, C.; O’Rourke, N.; Coyne, R.; Caher, A.; Mannion, A. Comorbid Psychopathology, Challenging Behavior, Sensory Issues, Adaptive Behavior and Quality of Life in Children and Adolescents with Autism Spectrum Disorder. Dev. Neurorehabil. 2021, 24, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Gomez, J.L.; De la Fuente-Anuncibay, R.R.; Vidriales-Fernandez, R.; Ortega-Camarero, M.T. The quality of life of people with ASD through physical activity and sports. Heliyon 2022, 8, e09193. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126. [Google Scholar]
- Ignacio, Z.M.; da Silva, R.S.; Plissari, M.E.; Quevedo, J.; Reus, G.Z. Physical Exercise and Neuroinflammation in Major Depressive Disorder. Mol. Neurobiol. 2019, 56, 8323–8335. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Barros, L.; Lima, A.B.; Nunes, T.; Carvalho, H.M.; Gaspar, J.M. Neuroinflammation in autism spectrum disorders: Exercise as a “pharmacological” tool. Neurosci. Biobehav. Rev. 2021, 129, 63–74. [Google Scholar] [CrossRef]
- Toscano, C.V.A.; Carvalho, H.M.; Ferreira, J.P. Exercise Effects for Children with Autism Spectrum Disorder: Metabolic Health, Autistic Traits, and Quality of Life. Percept. Mot. Ski. 2018, 125, 126–146. [Google Scholar] [CrossRef]
- Geslak, D.S.; Boudreaux, B.D. Exercise is a Life-Changer for those with autism. ACSM’s Health Fit. J. 2021, 25, 12–19. [Google Scholar] [CrossRef]
- Sowa, M.; Meulenbroek, R. Effects of physical exercise on autism spectrum disorders: A meta-analysis. Res. Autism Spectr. Disord. 2012, 6, 46–57. [Google Scholar] [CrossRef]
- Teh, E.J.; Vijayakumar, R.; Tan, T.X.J.; Yap, M.J. Effects of Physical Exercise Interventions on Stereotyped Motor Behaviours in Children with ASD: A Meta-Analysis. J. Autism Dev. Disord. 2022, 52, 2934–2957. [Google Scholar] [CrossRef]
- Petrus, C.; Adamson, S.R.; Block, L.; Einarson, S.J.; Sharifnejad, M.; Harris, S.R. Effects of exercise interventions on stereotypic behaviours in children with autism spectrum disorder. Physiother. Can. 2008, 60, 134–145. [Google Scholar] [CrossRef]
- Liang, X.; Li, R.; Wong, S.H.S.; Sum, R.K.W.; Wang, P.; Yang, B.; Sit, C.H.P. The Effects of Exercise Interventions on Executive Functions in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Sport. Med. 2022, 52, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Bremer, E.; Crozier, M.; Lloyd, M. A systematic review of the behavioural outcomes following exercise interventions for children and youth with autism spectrum disorder. Autism 2016, 20, 899–915. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Ghiarone, T.; Junior, C.R.C.; Furtado, G.E.; Carvalho, H.M.; Rodrigues, A.M.; Toscano, C.V.A. Effects of Physical Exercise on the Stereotyped Behavior of Children with Autism Spectrum Disorders. Medicina 2019, 55, 685. [Google Scholar] [CrossRef]
- Schmitz Olin, S.; McFadden, B.A.; Golem, D.L.; Pellegrino, J.K.; Walker, A.J.; Sanders, D.J.; Arent, S.M. The Effects of Exercise Dose on Stereotypical Behavior in Children with Autism. Med. Sci. Sport. Exerc. 2017, 49, 983–990. [Google Scholar] [CrossRef]
- Oriel, K.N.; Kanupka, J.W.; DeLong, K.S.; Noel, K. The impact of aquatic exercise on sleep behaviors in children with autism spectrum disorder: A pilot study. Focus Autism Other Dev. Disabil. 2016, 31, 254–261. [Google Scholar] [CrossRef]
- Yilmaz, I.; Yanardağ, M.; Birkan, B.; Bumin, G. Effects of swimming training on physical fitness and water orientation in autism. Pediatr. Int. 2004, 46, 624–626. [Google Scholar] [CrossRef]
- Pan, C.Y. Effects of water exercise swimming program on aquatic skills and social behaviors in children with autism spectrum disorders. Autism 2010, 14, 9–28. [Google Scholar] [CrossRef]
- Pitetti, K.H.; Rendoff, A.D.; Grover, T.; Beets, M.W. The efficacy of a 9-month treadmill walking program on the exercise capacity and weight reduction for adolescents with severe autism. J. Autism Dev. Disord. 2007, 37, 997–1006. [Google Scholar] [CrossRef]
- Oriel, K.N.; George, C.L.; Peckus, R.; Semon, A. The effects of aerobic exercise on academic engagement in young children with autism spectrum disorder. Pediatr. Phys. Ther. 2011, 23, 187–193. [Google Scholar] [CrossRef]
- Todd, T.; Reid, G.; Butler-Kisber, L. Cycling for students with ASD: Self-regulation promotes sustained physical activity. Adapt. Phys. Act. Q. 2010, 27, 226–241. [Google Scholar] [CrossRef]
- Koenig, K.P.; Buckley-Reen, A.; Garg, S. Efficacy of the Get Ready to Learn yoga program among children with autism spectrum disorders: A pretest-posttest control group design. Am. J. Occup. Ther. 2012, 66, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, L.E.; Gorantla, S.; Torres, J.A.; Yarmush, R.S.; Rao, S.; Park, E.R.; Denninger, J.W.; Benson, H.; Fricchione, G.L.; Bernstein, B.; et al. Relaxation response-based yoga improves functioning in young children with autism: A pilot study. J. Altern. Complement Med. 2011, 17, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Movahedi, A.; Marandi, S.M.; Abedi, A. Kata techniques training consistently decreases stereotypy in children with autism spectrum disorder. Res. Dev. Disabil. 2012, 33, 1183–1193. [Google Scholar] [CrossRef]
- Bahrami, F.; Movahedi, A.; Marandi, S.M.; Sorensen, C. The Effect of Karate Techniques Training on Communication Deficit of Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2016, 46, 978–986. [Google Scholar] [CrossRef]
- Movahedi, A.; Bahrami, F.; Marandi, S.M.; Abedi, A. Improvement in social dysfunction of children with autism spectrum disorder following long term Kata techniques training. Res. Autism Spectr. Disord. 2013, 7, 1054–1061. [Google Scholar] [CrossRef]
- Borgi, M.; Loliva, D.; Cerino, S.; Chiarotti, F.; Venerosi, A.; Bramini, M.; Nonnis, E.; Marcelli, M.; Vinti, C.; De Santis, C.; et al. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1–9. [Google Scholar] [CrossRef]
- Gabriels, R.L.; Agnew, J.A.; Holt, K.D.; Shoffner, A.; Zhaoxing, P.; Ruzzano, S.; Clayton, G.H.; Mesibov, G. Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2012, 6, 578–588. [Google Scholar] [CrossRef]
- Carey, M.; Sheehan, D.; Healy, S.; Knott, F.; Kinsella, S. The Effects of a 16-Week School-Based Exercise Program on Anxiety in Children with Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2022, 19, 5471. [Google Scholar] [CrossRef]
- Tse, C.Y.A.; Lee, H.P.; Chan, K.S.K.; Edgar, V.B.; Wilkinson-Smith, A.; Lai, W.H.E. Examining the impact of physical activity on sleep quality and executive functions in children with autism spectrum disorder: A randomized controlled trial. Autism 2019, 23, 1699–1710. [Google Scholar] [CrossRef]
- Ji, C.; Yang, J.; Lin, L.; Chen, S. Executive Function Improvement for Children with Autism Spectrum Disorder: A Comparative Study between Virtual Training and Physical Exercise Methods. Children 2022, 9, 507. [Google Scholar] [CrossRef]
- Coffey, C.; Carey, M.; Kinsella, S.; Byrne, P.J.; Sheehan, D.; Lloyd, R.S. Exercise Programming for Children with Autism Spectrum Disorder: Recommendations for Strength and Conditioning Specialists. Strength Cond. J. 2021, 43, 64–74. [Google Scholar] [CrossRef]
- Healy, S.; Msetfi, R.; Gallagher, S. ‘Happy and a bit nervous’: The experiences of children with autism in physical education. Br. J. Learn. Disabil. 2013, 41, 222–228. [Google Scholar] [CrossRef]
- Menear, K.S.; Smith, S. Physical education for students with autism: Teaching tips and strategies. Teach. Except. Child. 2008, 40, 32–37. [Google Scholar] [CrossRef]
- Hume, K.; Wong, C.; Plavnick, J.; Schultz, T. Use of visual supports with young children with autism spectrum disorders. In Handbook of Early Intervention for Autism Spectrum Disorders; Springer: Berlin/Heidelberg, Germany, 2014; pp. 293–313. [Google Scholar]
- Stanish, H.; Curtin, C.; Must, A.; Phillips, S.; Maslin, M.; Bandini, L. Enjoyment, Barriers, and Beliefs about Physical Activity in Adolescents with and without Autism Spectrum Disorder. Adapt. Phys. Act. Q. 2015, 32, 302–317. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Krajmalnik-Brown, R.; Porazinska, D.L.; Weiss, S.J.; Knight, R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell 2013, 155, 1446–1448. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial population changes and their relationship with human health and disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Diaz, J.; Jorquera-Aguilera, C.; Rodriguez-Rodriguez, A.; Rodriguez-Rodriguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Bermon, S.; Petriz, B.; Kajeniene, A.; Prestes, J.; Castell, L.; Franco, O.L. The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 2015, 21, 70–79. [Google Scholar]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef] [PubMed]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Redinbo, M.R. The microbiota, chemical symbiosis, and human disease. J. Mol. Biol. 2014, 426, 3877–3891. [Google Scholar] [CrossRef]
- Viggiano, D.; Ianiro, G.; Vanella, G.; Bibbò, S.; Bruno, G.; Simeone, G.; Mele, G. Gut barrier in health and disease: Focus on childhood. Eur. Rev. Med. Pharm. Sci. 2015, 19, 1077–1085. [Google Scholar]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Ho, L.K.H.; Tong, V.J.W.; Syn, N.; Nagarajan, N.; Tham, E.H.; Tay, S.K.; Shorey, S.; Tambyah, P.A.; Law, E.C.N. Gut microbiota changes in children with autism spectrum disorder: A systematic review. Gut Pathog. 2020, 12, 6. [Google Scholar] [CrossRef]
- Iglesias-Vazquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H. A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 2010, 33, 2–22. [Google Scholar] [CrossRef]
- Lou, M.; Cao, A.; Jin, C.; Mi, K.; Xiong, X.; Zeng, Z.; Pan, X.; Qie, J.; Qiu, S.; Niu, Y.; et al. Deviated and early unsustainable stunted development of gut microbiota in children with autism spectrum disorder. Gut 2022, 71, 1588–1599. [Google Scholar] [CrossRef]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.-L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002, 35, S6–S16. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef]
- Finegold, S.M. Therapy and epidemiology of autism–clostridial spores as key elements. Med. Hypotheses 2008, 70, 508–511. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, C.; Knudsen, J.; Leutscher, P.D.C.; Lauritsen, M.B.; Nyegaard, M.; Hagstrom, S.; Sorensen, S. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic literature review. Gut Microbes 2020, 11, 1172–1187. [Google Scholar] [CrossRef]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef]
- Alamoudi, M.U.; Hosie, S.; Shindler, A.E.; Wood, J.L.; Franks, A.E.; Hill-Yardin, E.L. Comparing the Gut Microbiome in Autism and Preclinical Models: A Systematic Review. Front. Cell Infect. Microbiol. 2022, 12, 905841. [Google Scholar] [CrossRef]
- Andreo-Martinez, P.; Rubio-Aparicio, M.; Sanchez-Meca, J.; Veas, A.; Martinez-Gonzalez, A.E. A Meta-analysis of Gut Microbiota in Children with Autism. J. Autism Dev. Disord. 2022, 52, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabro, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio 2012, 3, e00261-11. [Google Scholar] [CrossRef]
- Molitoris, E.; Wexler, H.; Finegold, S. Sources and antimicrobial susceptibilities of Campylobacter gracilis and Sutterella wadsworthensis. Clin. Infect. Dis. 1997, 25, S264–S265. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
- Pulikkan, J.; Maji, A.; Dhakan, D.B.; Saxena, R.; Mohan, B.; Anto, M.M.; Agarwal, N.; Grace, T.; Sharma, V.K. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microb. Ecol. 2018, 76, 1102–1114. [Google Scholar] [CrossRef]
- Zurita, M.F.; Cardenas, P.A.; Sandoval, M.E.; Pena, M.C.; Fornasini, M.; Flores, N.; Monaco, M.H.; Berding, K.; Donovan, S.M.; Kuntz, T.; et al. Analysis of gut microbiome, nutrition and immune status in autism spectrum disorder: A case-control study in Ecuador. Gut Microbes 2020, 11, 453–464. [Google Scholar] [CrossRef]
- Bezawada, N.; Phang, T.H.; Hold, G.L.; Hansen, R. Autism Spectrum Disorder and the Gut Microbiota in Children: A Systematic Review. Ann. Nutr. Metab. 2020, 76, 16–29. [Google Scholar] [CrossRef]
- Kang, D.W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, M.; Feng, Y.; Zhou, Z.; Chen, L.; Chen, F. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci. Rep. 2018, 8, 1597. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children With Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci. Rep. 2018, 8, 13981. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Fernandez, A.; Chueca, N.; Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martin-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef]
- Zhang, M.; Chu, Y.; Meng, Q.; Ding, R.; Shi, X.; Wang, Z.; He, Y.; Zhang, J.; Liu, J.; Zhang, J.; et al. A quasi-paired cohort strategy reveals the impaired detoxifying function of microbes in the gut of autistic children. Sci. Adv. 2020, 6, eaba3760. [Google Scholar] [CrossRef]
- Wan, Y.; Zuo, T.; Xu, Z.; Zhang, F.; Zhan, H.; Chan, D.; Leung, T.F.; Yeoh, Y.K.; Chan, F.K.L.; Chan, R.; et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut 2022, 71, 910–918. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Karkkainen, O.; Laye, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Eltokhi, A.; Santuy, A.; Merchan-Perez, A.; Sprengel, R. Glutamatergic Dysfunction and Synaptic Ultrastructural Alterations in Schizophrenia and Autism Spectrum Disorder: Evidence from Human and Rodent Studies. Int. J. Mol. Sci. 2020, 22, 59. [Google Scholar] [CrossRef]
- Chen, J.; Yu, S.; Fu, Y.; Li, X. Synaptic proteins and receptors defects in autism spectrum disorders. Front. Cell Neurosci. 2014, 8, 276. [Google Scholar] [CrossRef]
- DiCarlo, G.E.; Mabry, S.J.; Cao, X.; McMillan, C.; Woynaroski, T.G.; Harrison, F.E.; Reddy, I.A.; Matthies, H.J.G.; Flynn, C.R.; Wallace, M.T.; et al. Autism-Associated Variant in the SLC6A3 Gene Alters the Oral Microbiome and Metabolism in a Murine Model. Front. Psychiatry 2021, 12, 655451. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, X.; Dan, Z.; Pei, Y.; Xu, R.; Guo, M.; Liu, K.; Zhang, F.; Chen, J.; Su, C.; et al. Gene variations in autism spectrum disorder are associated with alteration of gut microbiota, metabolites and cytokines. Gut Microbes 2021, 13, 1854967. [Google Scholar] [CrossRef]
- Knights, D.; Silverberg, M.S.; Weersma, R.K.; Gevers, D.; Dijkstra, G.; Huang, H.; Tyler, A.D.; Van Sommeren, S.; Imhann, F.; Stempak, J.M. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014, 6, 107. [Google Scholar] [CrossRef]
- Kolde, R.; Franzosa, E.A.; Rahnavard, G.; Hall, A.B.; Vlamakis, H.; Stevens, C.; Daly, M.J.; Xavier, R.J.; Huttenhower, C. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome Med. 2018, 10, 6. [Google Scholar] [CrossRef]
- Patusco, R.; Ziegler, J. Role of Probiotics in Managing Gastrointestinal Dysfunction in Children with Autism Spectrum Disorder: An Update for Practitioners. Adv. Nutr. 2018, 9, 637–650. [Google Scholar] [CrossRef]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Kung, J.Y.; Tun, H.M.; Wine, E.; Madsen, K.L.; Zwaigenbaum, L.; Haqq, A.M. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: A systematic review. Autism Res. 2021, 14, 1820–1836. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Mishra, D.; Eshraghi, R.S.; Mittal, J.; Sinha, R.; Bulut, E.; Mittal, R.; Eshraghi, A.A. Altering the gut microbiome to potentially modulate behavioral manifestations in autism spectrum disorders: A systematic review. Neurosci. Biobehav. Rev. 2021, 128, 549–557. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, J.; Chang, L.; Qu, Y.; Wang, S.; Zhang, K.; Xiong, Z.; Zhang, J.; Tan, Y.; Wang, X.; et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2020, 117, 11753–11759. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharm. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Guidetti, C.; Salvini, E.; Viri, M.; Deidda, F.; Amoruso, A.; Visciglia, A.; Drago, L.; Calgaro, M.; Vitulo, N.; Pane, M. Randomized Double-Blind Crossover Study for Evaluating a Probiotic Mixture on Gastrointestinal and Behavioral Symptoms of Autistic Children. J. Clin. Med. 2022, 11, 5263. [Google Scholar] [CrossRef]
- Duque, A.; Demarqui, F.M.; Santoni, M.M.; Zanelli, C.F.; Adorno, M.A.T.; Milenkovic, D.; Mesa, V.; Sivieri, K. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Res. Int. 2021, 149, 110657. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejia, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Mohammad, F.K.; Palukuri, M.V.; Shivakumar, S.; Rengaswamy, R.; Sahoo, S. A Computational Framework for Studying Gut-Brain Axis in Autism Spectrum Disorder. Front. Physiol. 2022, 13, 760753. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Timperio, A.M.; Gevi, F.; Cucinotta, F.; Ricciardello, A.; Turriziani, L.; Scattoni, M.L.; Persico, A.M. Urinary Untargeted Metabolic Profile Differentiates Children with Autism from Their Unaffected Siblings. Metabolites 2022, 12, 797. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Teng, L.; Wang, Y.; Zhu, L. Prebiotics and probiotics for autism spectrum disorder: A systematic review and meta-analysis of controlled clinical trials. J. Med. Microbiol. 2022, 71, 001510. [Google Scholar] [CrossRef]
- Adams, J.B.; Borody, T.J.; Kang, D.W.; Khoruts, A.; Krajmalnik-Brown, R.; Sadowsky, M.J. Microbiota transplant therapy and autism: Lessons for the clinic. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 1033–1037. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, e00314-20. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell Infect Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Zhong, H.J.; Huang, D.N.; Wu, L.H.; He, X.X. Beneficial Effects of Repeated Washed Microbiota Transplantation in Children With Autism. Front. Pediatr. 2022, 10, 928785. [Google Scholar] [CrossRef]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Oines, M.N.; Wiig, H.; Rose, O.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef]
- Mitchell, L.K.; Davies, P.S.W. Pre- and probiotics in the management of children with autism and gut issues: A review of the current evidence. Eur. J. Clin. Nutr. 2022, 76, 913–921. [Google Scholar] [CrossRef]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef]
- Won, H.; Mah, W.; Kim, E. Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Front. Mol. Neurosci. 2013, 6, 19. [Google Scholar] [CrossRef]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Marshall, C.R.; Noor, A.; Vincent, J.B.; Lionel, A.C.; Feuk, L.; Skaug, J.; Shago, M.; Moessner, R.; Pinto, D.; Ren, Y. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008, 82, 477–488. [Google Scholar] [CrossRef]
- Berkel, S.; Marshall, C.R.; Weiss, B.; Howe, J.; Roeth, R.; Moog, U.; Endris, V.; Roberts, W.; Szatmari, P.; Pinto, D. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010, 42, 489–491. [Google Scholar] [CrossRef]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Ercument Cicek, A.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Gilman, S.R.; Iossifov, I.; Levy, D.; Ronemus, M.; Wigler, M.; Vitkup, D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 2011, 70, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.-P.; Wilkerson, J.R.; Guo, W.; Maksimova, M.A.; DeMartino, G.N.; Cowan, C.W.; Huber, K.M. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 2012, 151, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet 2014, 10, e1004580. [Google Scholar] [CrossRef]
- Arnett, A.B.; Trinh, S.; Bernier, R.A. The state of research on the genetics of autism spectrum disorder: Methodological, clinical and conceptual progress. Curr. Opin. Psychol. 2019, 27, 1–5. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e523. [Google Scholar] [CrossRef]
- Cheng, D.; Hoogenraad, C.C.; Rush, J.; Ramm, E.; Schlager, M.A.; Duong, D.M.; Xu, P.; Wijayawardana, S.R.; Hanfelt, J.; Nakagawa, T. Relative and Absolute Quantification of Postsynaptic Density Proteome Isolated from Rat Forebrain and Cerebellum* S. Mol. Cell. Proteom. 2006, 5, 1158–1170. [Google Scholar] [CrossRef]
- Cho, K.-O.; Hunt, C.A.; Kennedy, M.B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron 1992, 9, 929–942. [Google Scholar] [CrossRef]
- Kaizuka, T.; Takumi, T. Postsynaptic density proteins and their involvement in neurodevelopmental disorders. J. Biochem. 2018, 163, 447–455. [Google Scholar] [CrossRef]
- Zeng, M.; Shang, Y.; Guo, T.; He, Q.; Yung, W.H.; Liu, K.; Zhang, M. A binding site outside the canonical PDZ domain determines the specific interaction between Shank and SAPAP and their function. Proc. Natl. Acad. Sci. USA 2016, 113, E3081–E3090. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.J. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Iasevoli, F.; Buonaguro, E.F.; De Berardis, D.; Fornaro, M.; Fiengo, A.L.; Martinotti, G.; Orsolini, L.; Valchera, A.; Di Giannantonio, M.; et al. Treating the Synapse in Major Psychiatric Disorders: The Role of Postsynaptic Density Network in Dopamine-Glutamate Interplay and Psychopharmacologic Drugs Molecular Actions. Int. J. Mol. Sci. 2017, 18, 135. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, T.-X.; Hallett, P.J.; Watanabe, M.; Grant, S.G.; Isacson, O.; Yao, W.-D. PSD-95 uncouples dopamine–glutamate interaction in the D1/PSD-95/NMDA receptor complex. J. Neurosci. 2009, 29, 2948–2960. [Google Scholar] [CrossRef]
- Gu, W.H.; Yang, S.; Shi, W.X.; Jin, G.Z.; Zhen, X.C. Requirement of PSD-95 for dopamine D1 receptor modulating glutamate NR1a/NR2B receptor function 1. Acta Pharmacol. Sin. 2007, 28, 756–762. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Horrocks, M.H.; Zhu, F.; Muresan, L.; Benavides-Piccione, R.; DeFelipe, J.; Fricker, D.; Kopanitsa, M.V.; Duncan, R.R.; Klenerman, D.; et al. PSD95 nanoclusters are postsynaptic building blocks in hippocampus circuits. Sci. Rep. 2016, 6, 24626. [Google Scholar] [CrossRef]
- Nagura, H.; Ishikawa, Y.; Kobayashi, K.; Takao, K.; Tanaka, T.; Nishikawa, K.; Tamura, H.; Shiosaka, S.; Suzuki, H.; Miyakawa, T. Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice. Mol. Brain 2012, 5, 43. [Google Scholar] [CrossRef]
- Feyder, M.; Karlsson, R.-M.; Mathur, P.; Lyman, M.; Bock, R.; Momenan, R.; Munasinghe, J.; Scattoni, M.L.; Ihne, J.; Camp, M. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams’ syndrome. Am. J. Psychiatry 2010, 167, 1508–1517. [Google Scholar] [CrossRef]
- Naisbitt, S.; Kim, E.; Tu, J.C.; Xiao, B.; Sala, C.; Valtschanoff, J.; Weinberg, R.J.; Worley, P.F.; Sheng, M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999, 23, 569–582. [Google Scholar] [CrossRef]
- Kuriu, T.; Inoue, A.; Bito, H.; Sobue, K.; Okabe, S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J. Neurosci. 2006, 26, 7693–7706. [Google Scholar] [CrossRef]
- Sudhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Arons, M.H.; Thynne, C.J.; Grabrucker, A.M.; Li, D.; Schoen, M.; Cheyne, J.E.; Boeckers, T.M.; Montgomery, J.M.; Garner, C.C. Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling. J. Neurosci. 2012, 32, 14966–14978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zu, X.; Luo, W.; Yang, H.; Luo, G.; Zhang, M.; Tang, S. Social isolation produces anxiety-like behaviors and changes PSD-95 levels in the forebrain. Neurosci. Lett. 2012, 514, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Mack, N.R. From Hyposociability to Hypersociability-The Effects of PSD-95 Deficiency on the Dysfunctional Development of Social Behavior. Front. Behav. Neurosci. 2021, 15, 618397. [Google Scholar] [CrossRef]
- Winkler, D.; Daher, F.; Wüstefeld, L.; Hammerschmidt, K.; Poggi, G.; Seelbach, A.; Krueger-Burg, D.; Vafadari, B.; Ronnenberg, A.; Liu, Y. Hypersocial behavior and biological redundancy in mice with reduced expression of PSD95 or PSD93. Behav. Brain Res. 2018, 352, 35–45. [Google Scholar] [CrossRef]
- Sontheimer, H. Diseases of Motor Neurons and Neuromuscular Junctions. Dis. Nerv. Syst. 2021, 2, 135. [Google Scholar]
- Littrell, J. Neuroscience for Psychologists and Other Mental Health Professionals: Promoting Well-Being and Treating Mental Illness; Springer Publishing Company: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Xu, F.; Fu, Y.; Sun, T.Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.W.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef]
| Types of Physical Activity | Clinical Effect | Reference |
|---|---|---|
| Aquatic exercises | Improvements in sleep latency and duration | [79,80,81] |
| Walking/Running | Improvements in weight management and academic engagement | [82,83] |
| Cycling | Improvements in self-regulation and physical activity | [84] |
| Yoga and dance | Improvements in classroom behaviors, behavioral symptoms, and depression | [85,86] |
| Martial Arts | Improvements in stereotypy and communication deficits | [87,88,89] |
| Horseback riding | Improvements in executive function, irritability, and stereotypic behaviour | [90,91] |
| Fundamental movement skills | Improvements in anxiety symptoms | [92] |
| Basketball | Improvements in sleep quality | [93] |
| Football-based training | Improvements in executive function | [94] |
| Biological Material | Microbes | Clinical Impact | References |
|---|---|---|---|
| Stool samples | ASD group had greater levels of Bacteroidetes, while the control group had greater levels of Firmicutes. Both Actinobacterium and Proteobacterium displayed smaller but significant differences. The prevalence of Desulfovibrio species and Bacteroides vulgatus in the stools of children with ASD is significantly higher than that in children without ASD. | Describing microbial signatures related with health | [114] |
| Ileal and cecal samples | Higher prevalence of Sutterella spp. | Children with ASD and gastrointestinal dysfunction have a significantly higher level of Sutterella present in their microbiota than children with only gastrointestinal dysfunction. | [128] |
| Stool samples | The number of Sutterella spp. in the feces of children with ASD is higher than that of controls. | The number of Ruminococcus torques is also higher in those children with ASD who have been diagnosed with a functional gastrointestinal disorder. | [130] |
| Stool samples | The Firmicutes/Bacteroidetes ratio significantly increased in patients with ASD due to a reduction in Bacteroidetes. Among the cohort with ASD, Alistipes, Bilophila, Dialister, Parabacteroides, and Veillonella decreased in relative abundance, while Collinsella, Corynebacterium, Dorea, and Lactobacillus increased. Children with ASD have a higher level of Clostridium cluster XVIII and Escherichia/Shigella. Candida abundances was also significantly different in ASD subjects compared to healthy subjects. | ASDs are associated with altered intestinal microbial communities at both the bacterial and fungal levels. This is not determined by the constipation status of ASD individuals, but rather by the ASD itself. | [127] |
| Stool samples | Children with ASD also had lower abundances of Haemophilus parainfluenzae and Faecalibacterium prausnitzii in their feces after multiple testing corrections. | The results obtained in this study suggest that children with ASD have different metabolite profiles in their feces. | [134] |
| Dental and salivary samples | ASD patients’ saliva and plaques contained increased levels of Haemophilus and Streptococcus. As a result, commensal bacteria Selenomonas, Prevotella, Actinomyces, Fusobacterium, and Porphyromonas were reduced in number. | Clinical indices, such as severity of disease and oral health, were also correlated with distinguishable bacteria. | [136] |
| Stool samples | Children with ASD have a gut microbiota that consists predominantly of Bifidobacteraceae, Lactobacillaceae, and Veillonellaceae; healthy children have a gut microbiota that consists primarily of Prevotellaceae. | In this study, differences in microbial community structure were identified between children with ASD and healthy children. | [131] |
| Stool samples | In the fecal microbiota of the ASD group, the Bacteroidetes/Firmicutes ratio increased significantly. The relative abundance of Sutterella, Odoribacter, and Butyricimonas was significantly higher in the ASD group as compared to the control group, whereas Veillonella and Streptococcus were significantly decreased. | ASD showed a positive correlation with periodontal disease and a negative correlation with type 1 diabetes in this microbe-disease network based on microbe similarity of diseases. | [139] |
| Stool samples | There was an increase in Acidobacteria but a decrease in Ruminococcaceae, Eubacterium, Lachnospiraceae, and Erysipelotrichaceae among ASD individuals. | ASD may be treatable by modulating the gut microbiota. | [140] |
| Stool samples | There was a much higher proportion of Proteobacteria and Actinobacteria in children with ASD, as well as Bacilli, Actinobacteria, Erysipelotrichi, and Gammaproteobacteria in the class of children with ASD. | In children with ASD, mixed bacterial and nutritional variables showed differential patterns. | [141] |
| Stool samples | In children with ASD, Bacteroides, Coprococcus, Akkermansia, and Ruminococcus spp. were more abundant and diverse. | Children with ASD have abnormal eating habits, as well as common gastrointestinal symptoms that are associated with nutritional differences. | [132] |
| Saliva and stool samples | Bacilli was significantly higher in the gut of ASD individuals compared to controls. | ASD and comorbid conditions can be diagnosed and treated using microbial markers. | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Diaz, J.; Radar, A.M.; Baig, A.T.; Leyba, M.F.; Costabel, M.M.; Zavala-Crichton, J.P.; Sanchez-Martinez, J.; MacKenzie, A.E.; Solis-Urra, P. Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder. Children 2022, 9, 1834. https://doi.org/10.3390/children9121834
Plaza-Diaz J, Radar AM, Baig AT, Leyba MF, Costabel MM, Zavala-Crichton JP, Sanchez-Martinez J, MacKenzie AE, Solis-Urra P. Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder. Children. 2022; 9(12):1834. https://doi.org/10.3390/children9121834
Chicago/Turabian StylePlaza-Diaz, Julio, Ana Mei Radar, Aiman Tariq Baig, Marcos Federico Leyba, Maria Macarena Costabel, Juan Pablo Zavala-Crichton, Javier Sanchez-Martinez, Alex E. MacKenzie, and Patricio Solis-Urra. 2022. "Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder" Children 9, no. 12: 1834. https://doi.org/10.3390/children9121834
APA StylePlaza-Diaz, J., Radar, A. M., Baig, A. T., Leyba, M. F., Costabel, M. M., Zavala-Crichton, J. P., Sanchez-Martinez, J., MacKenzie, A. E., & Solis-Urra, P. (2022). Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder. Children, 9(12), 1834. https://doi.org/10.3390/children9121834








