CDKL5 Deficiency Disorder (CDD)—Rare Presentation in Male
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahi-Buisson, N.; Bienvenu, T. CDKL5-Related Disorders: From Clinical Description to Molecular Genetics. Mol. Syndr. 2011, 2, 137–152. [Google Scholar] [CrossRef]
- Barbiero, I.; De Rosa, R.; Kilstrup-Nielsen, C. Microtubules: A Key to Understand and Correct Neuronal Defects in CDKL5 Deficiency Disorder? Int. J. Mol. Sci. 2019, 20, 4075. [Google Scholar] [CrossRef] [PubMed]
- Terzic, B.; Davatolhagh, M.F.; Ho, Y.; Tang, S.; Liu, Y.-T.; Xia, Z.; Cui, Y.; Fuccillo, M.V.; Zhou, Z. Temporal manipulation of Cdkl5 reveals essential postdevelopmental functions and reversible CDKL5 deficiency disorder–related deficits. J. Clin. Investig. 2021, 131, e143655. [Google Scholar] [CrossRef]
- Olson, H.E.; Demarest, S.T.; Pestana-Knight, E.M.; Swanson, L.C.; Iqbal, S.; Lal, D.; Leonard, H.; Cross, H.; Devinsky, O.; Benke, T.A. Cyclin-Dependent Kinase-Like 5 Deficiency Disorder: Clinical Review. Pediatr. Neurol. 2019, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fehr, S.; Wilson, M.; Downs, J.; Williams, S.J.; Murgia, A.; Sartori, S.; Vecchi, M.; Ho, G.; Polli, R.; Psoni, S.; et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur. J. Hum. Genet. 2012, 21, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Fehr, S.; Downs, J.; Ho, G.; de Klerk, N.; Forbes, D.; Christodoulou, J.; Williams, S.; Leonard, H. Functional abilities in children and adults with the CDKL5 disorder. Am. J. Med. Genet. Part A 2016, 170, 2860–2869. [Google Scholar] [CrossRef]
- Jakimiec, M.; Paprocka, J.; Śmigiel, R. CDKL5 Deficiency Disorder—A Complex Epileptic Encephalopathy. Brain Sci. 2020, 10, 107. [Google Scholar] [CrossRef]
- Mirzaa, G.M.; Paciorkowski, A.R.; Marsh, E.D.; Berry-Kravis, E.M.; Medne, L.; Grix, A.; Wirrell, E.C.; Powell, B.R.; Nickels, K.C.; Burton, B.; et al. CDKL5 and ARX Mutations in Males With Early-Onset Epilepsy. Pediatr. Neurol. 2013, 48, 367–377. [Google Scholar] [CrossRef]
- Leonard, H.; Downs, J.; Benke, T.A.; Swanson, L.; Olson, H.; Demarest, S. CDKL5 deficiency disorder: Clinical features, diagnosis, and management. Lancet Neurol. 2022, 21, 563–576. [Google Scholar] [CrossRef]
- Lindy, A.S.; Stosser, M.B.; Butler, E.; Downtain-Pickersgill, C.; Shanmugham, A.; Retterer, K.; Brandt, T.; Richard, G.; McKnight, D.A. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018, 59, 1062–1071. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Anesthesia Analg. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Liang, J.-S.; Huang, H.; Wang, J.-S.; Lu, J.-F. Phenotypic manifestations between male and female children with CDKL5 mutations. Brain Dev. 2019, 41, 783–789. [Google Scholar] [CrossRef]
- Siri, B.; Varesio, C.; Freri, E.; Darra, F.; Gana, S.; Mei, D.; Porta, F.; Fontana, E.; Galati, G.; Solazzi, R.; et al. CDKL5 deficiency disorder in males: Five new variants and review of the literature. Eur. J. Paediatr. Neurol. 2021, 33, 9–20. [Google Scholar] [CrossRef]
- Weaving, L.S.; Christodoulou, J.; Williamson, S.L.; Friend, K.L.; McKenzie, O.L.; Archer, H.; Evans, J.; Clarke, A.; Pelka, G.J.; Tam, P.P.; et al. Mutations of CDKL5 Cause a Severe Neurodevelopmental Disorder with Infantile Spasms and Mental Retardation. Am. J. Hum. Genet. 2004, 75, 1079–1093. [Google Scholar] [CrossRef]
- Neupauerová, J.; Štěrbová, K.; Vlčková, M.; Sebroňová, V.; Maříková, T.; Krůtová, M.; David, S.; Kršek, P.; Žaliová, M.; Seeman, P.; et al. Two Novel Variants Affecting CDKL5 Transcript Associated with Epileptic Encephalopathy. Genet. Test. Mol. Biomarkers 2017, 21, 613–618. [Google Scholar] [CrossRef]
- Bartnik, M.; Derwińska, K.; Gos, M.; Obersztyn, E.; Kołodziejska, K.E.; Erez, A.; Szpecht-Potocka, A.; Fang, P.; Terczyńska, I.; Mierzewska, H.; et al. Early-onset seizures due to mosaic exonic deletions of CDKL5 in a male and two females. Genet. Med. 2011, 13, 447–452. [Google Scholar] [CrossRef]
- Arafat, A.; Jing, P.; Ma, Y.; Pu, M.; Nan, G.; Fang, H.; Chen, C.; Fei, Y. Unexplained Early Infantile Epileptic Encephalopathy in Han Chinese Children: Next-Generation Sequencing and Phenotype Enriching. Sci. Rep. 2017, 7, 46227. [Google Scholar] [CrossRef]
- Castrén, M.; Gaily, E.; Tengström, C.; Lähdetie, J.; Archer, H.; Ala-Mello, S. Epilepsy caused by CDKL5 mutations. Eur. J. Paediatr. Neurol. 2011, 15, 65–69. [Google Scholar] [CrossRef]
- Mei, D.; Darra, F.; Barba, C.; Marini, C.; Fontana, E.; Chiti, L.; Parrini, E.; Bernardina, B.D.; Guerrini, R. Optimizing the molecular diagnosis of CDKL5 gene-related epileptic encephalopathy in boys. Epilepsia 2014, 55, 1748–1753. [Google Scholar] [CrossRef]
- Liang, J.-S.; Shimojima, K.; Takayama, R.; Natsume, J.; Shichiji, M.; Hirasawa, K.; Imai, K.; Okanishi, T.; Mizuno, S.; Okumura, A.; et al. CDKL5 alterations lead to early epileptic encephalopathy in both genders. Epilepsia 2011, 52, 1835–1842. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. ClinVar; [VCV000143822.8]. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000143822.8 (accessed on 24 October 2022).
- Lim, Z.; Wong, K.; Olson, H.E.; Bergin, A.M.; Downs, J.; Leonard, H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: Experience of >100 patients. Epilepsia 2017, 58, 1415–1422. [Google Scholar] [CrossRef]
- Lim, Z.; Wong, K.; Downs, J.; Bebbington, K.; Demarest, S.; Leonard, H. Vagus nerve stimulation for the treatment of refractory epilepsy in the CDKL5 Deficiency Disorder. Epilepsy Res. 2018, 146, 36–40. [Google Scholar] [CrossRef]
- Toffa, D.H.; Touma, L.; El Meskine, T.; Bouthillier, A.; Nguyen, D.K. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: A critical review. Seizure 2020, 83, 104–123. [Google Scholar] [CrossRef]
- Orosz, I.; McCormick, D.; Zamponi, N.; Varadkar, S.; Feucht, M.; Parain, D.; Griens, R.; Vallée, L.; Boon, P.; Rittey, C.; et al. Vagus nerve stimulation for drug-resistant epilepsy: A European long-term study up to 24 months in 347 children. Epilepsia 2014, 55, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/media/106046/download (accessed on 24 August 2022).
- Demarest, S.T.; Olson, H.E.; Moss, A.; Pestana-Knight, E.; Zhang, X.; Parikh, S.; Swanson, L.C.; Riley, K.D.; Bazin, G.A.; Angione, K.; et al. CDKL5 deficiency disorder: Relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia 2019, 60, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Mangatt, M.; Wong, K.; Anderson, B.; Epstein, A.; Hodgetts, S.; Leonard, H.; Downs, J. Prevalence and onset of comorbidities in the CDKL5 disorder differ from Rett syndrome. Orphanet, J. Rare Dis. 2016, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.M.P.; Amin, S.; Bahi-Buisson, N.; Benke, T.A.; Cross, J.H.; Demarest, S.T.; Olson, H.E.; Specchio, N.; Fleming, T.R.; Aimetti, A.A.; et al. Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: Results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2022, 21, 417–427. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Drug for Treatment of Seizures Associated with Rare Disease in Patients Two Years of Age and Older. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treatment-seizures-associated-rare-disease-patients-two-years-age-and-older (accessed on 24 August 2022).
- Olson, H.E.; Daniels, C.I.; Haviland, I.; Swanson, L.C.; Greene, C.A.; Denny, A.M.M.; Demarest, S.T.; Pestana-Knight, E.; Zhang, X.; Moosa, A.N.; et al. Current neurologic treatment and emerging therapies in CDKL5 deficiency disorder. J. Neurodev. Disord. 2021, 13, 40. [Google Scholar] [CrossRef]
- Gao, Y.; Irvine, E.E.; Eleftheriadou, I.; Naranjo, C.J.; Hearn-Yeates, F.; Bosch, L.; Glegola, J.A.; Murdoch, L.; Czerniak, A.; Meloni, I.; et al. Gene replacement ameliorates deficits in mouse and human models of cyclin-dependent kinase-like 5 disorder. Brain 2020, 143, 811–832. [Google Scholar] [CrossRef]
| Subject | CDKL5 Variant | Type of Variant | Inheritance | Seizure Onset (Age) | Type of Seizure | EEG Features |
|---|---|---|---|---|---|---|
| 1 Weaving, L. S. et al., 2004 [14] | c.183del T p.Met63CysfsX13 | Deletion | Parental mosaicism (mother) | 4 weeks | Epileptic spasms | Hypsarrhythmia |
| 2 Castrén, M. et al., 2011 [18] | del. 0.3 Mb | Deletion | De novo | 5 weeks | Generalized tonic, epileptic spasms, myoclonic | Slow waves and multifocal spasm, diffuse slow waves; multifocal spikes; EEG showed multifocal spikes during sleep |
| 3 Liang, J. S. et al., 2011 [20] | 5′UTR region (Xp22.13—no band for exon 1B, thus confirming nullisomy of this region) | Deletion | De novo | 1 month | Epileptic spasms | Not available |
| 4 Bartnik, M. et al., 2011 [16] | Xp22.13 del.105 kb | Deletion | De novo | 6 weeks | Secondary generalized seizures | Interictal: abnormal slowing of occipital dominant rhythm, multifocal sharp waves, left frontal and central spikes and waves |
| Subject | CDKL5 variant | Type of Variant | Inheritance | Seizure Onset (age) | Type of Seizure | EEG Features |
| 5 Mirzaa, G.M. et al., 2013 [8] | c.578A > G p.Asp193Gly | Missense | Germline mosaicism | 4 weeks | Tonic, atonic, myoclonic | Hypsarrhythmia; multifocal polyspikes, discontinuous background and periodic burst suppression |
| 6 Mei, D. et al., 2014 [19] | Deletion exon 1 | Promoter full deletion (in frame-deletion) | De novo full hemizygous deletion | 1 months | Tonic seizures, epileptic spasms | Multifocal paroxysmal activity |
| 7 Arafat, A. et al., 2017 [17] | c.278dupA p.Asn95LysfsX16 | Frameshift | De novo | 1 month | Focal epileptic spasms | Hypsarrhythmia |
| 8 Neupauerova, J. et al., 2017 [15] | c.2578C > T p.Gln860X | Missense | De novo | 1 month | Hypermotor seizures, epileptic spasms, myoclonic, tonic seizures | Normal; then right-sided fronto-temporal spikes; hypsarrhytmia |
| 9 Siri, B. et al., 2021 [13] | c.601_603delCTT p.Leu201del | In-frame deletion | De novo | 1 month | Epileptic spasms, focal to bilateral tonic–clonic, focal motor, tonic seizures | Slowing of background activity, unilateral and bilateral focal low-voltage rapid rhythmic activity, followed by discharge of rhythmic spikes and waves or polyspikes; hypsarrhytmia |
| Subject | CDKL5 Variant | Type of Variant | Inheritance | Seizure Onset (age) | Type of Seizure | EEG Features |
| 10 Siri, B. et al., 2021 [13] | c.825þ1G > T | Splice site (within intron 10) | De novo | 25 days | Focal impairned awareness, epileptic spasms and tonic, myoclonic, hypermotor seizures | Slowing of background activity, waves and spikes and waves on left parieto- temporal regions and diffuse spikes and waves, recruiting diffuse discharges on left fronto-parietal cortex; multifocal spikes on parietal region, atypical hypsarrythmia |
| 11 Mirzaa, G.M. et al., 2013 [8] | c.513C > A p.Tyr171X (exon 8) | Nonsense | De novo | 5.5 weeks | Tonic, tonic–clonic, generalized seizures, epileptic spasms | Hypsarrhythmia |
| 12 Mirzaa, G.M. et al., 2013 [8] | c.175C > T p.Arg59X (exon 5) | Nonsense | De novo | 6 weeks | Epileptic spasms, myoclonic, tonic seizures | Continuous bihemispheric, epileptiform discharges |
| 13 Mirzaa, G.M. et al., 2013 [8] | c.2593C > T p.Gln865X (exon 13) | Nonsense | De novo | 1.5 months | Epileptic spasms, focal, multifocal seizures | Multifocal, epileptiform discharges; recorded generalized seizures |
| 14 Mirzaa, G.M. et al., 2013 [8] | Deletion exon 3 | Deletion | De novo | 2 weeks | Epileptic spasms, tonic, tonic–clonic, myoclonic seizures | Slow R sharp waves; disorganized, slow, sharp waves, multifocal discharges |
| 15 Mirzaa, G.M. et al., 2013 [8] | c.62A > G p.Glu21Gly (exon 2) | Missense | De novo | 5.5 weeks | Epileptic spasms, tonic seizures | Localization related epilepsy, likely arising from the right frontocentral area |
| 16 The present study | c.513C > G p.Tyr171X | Nonsense | De novo | 5 weeks | Epileptic seizures, myoclonic, focal motor seizures | The first EEG showed generalized paroxysmal discharges, without the typical bioelectrical silence episodes with normal sleep characteristics. The second EEG showed continuous paroxysmal changes. During video EEG recording, limb tearing and eye rotations were observed |
| Subject | MRI | Eye Contact, Movement Disorders | Tone/Reflexes | Motor Skills | Comorbidities | Treatment |
| 1 Weaving, L. S. et al., 2004 [14] | Not available | Poor eye contact, movement disorders: severe global developmental delay | Spastic quadriparesis | None | Gastroesophageal reflux, constipation, pneumonia, kyphoscoliosis, hyperventilation | Seizures very difficult to control, despite numerous ASMs (the names of the medicines were not included in the article), trial of adrenocorticotropic hormone, and ketogenic diet |
| 2 Castrén, M. et al., 2011 [18] | Normal | Poor eye contact, movement disorders: severe global developmental delay | Hypotonia | None | Triangular face, facial dysmorphia, ligamentous laxity | Seizures very difficult to control, despite numerous ASMs (rufinamide, oxcarbazepine, clonazepam and levetiracetam), ketogenic diet resistance |
| Subject | MRI | Eye Contact, Movement Disorders | Tone/Reflexes | Motor Skills | Comorbidities | Treatment |
| 3 Liang, J. S. et al., 2011 [20] | Cerebral atrophy | Severe global developmental delay | Normal | None | Not available | ASM resistance (the names of the medicines were not included in the article) |
| 4 Bartnik, M. et al., 2011 [16] | Normal | Severe global developmental delay | Hypotonia, hyporeflexia | None | No speech | ASM resistance (phenobarbital, levetiracetam, lamotrigine, folinic acid, and pyridoxine sulfate), patient on a ketogenic diet (no information about its effectiveness) |
| 5 Mirzaa, G.M. et al., 2013 [8] | Normal | Poor eye contact, movement disorders: severe global developmental delay | Axial hypotonia Limb spasticity | None | Gastroesophageal reflux, constipation, scoliosis, facial dysmorphia, optic atrophy | Partial response to phenobarbital, carbamazepine, and vigabatrin, but the child was never seizure-free |
| 6 Mei, D. et al., 2014 [19] | Normal | Not available | Quadriparesis with hypotonia | Severe developmental delay | Not available | ASM resistance (the names of the medicines were not included in the article) |
| 7 Arafat, A. et al., 2017 [17] | Ventriculomegaly | Not available | Not available | Severe global developmental delay | Not available | ASMs (oxcarbazepine, carbamazepine, levetiracetam, phenobarbital, topiramate, sodium valproate) and ketogenic diet resistance |
| Subject | MRI | Eye Contact, Movement Disorders | Tone/Reflexes | Motor Skills | Comorbidities | Treatment |
| 8 Neupauerova, J. et al., 2017 [15] | Mild frontal atrophy | Poor eye contact, no movement disorder | Hypotonia | Severe global developmental delay | Tetralogy of Fallot, sleep problems | ASM (valproic acid, topiramate, phenobarbital, vigabatrin, levetiracetam, phenytoin, clobazam, and their combinations) resistance, partial response to ketogenic diet, adrenocorticotropic hormone partially effective |
| 9 Siri, B. et al., 2021 [13] | Subarachnoid space widening | Poor eye contact, movement disorder | Hypotonia | Severe global developmental delay, motor skills: none | Liquid dysphagia, laryngeal stridor, sleep problems, horizontal nystagmus | ASM (the names of the medicines were not included in the article) and ketogenic diet resistance |
| 10 Siri, B. et al., 2021 [13] | Widening of subarachnoid space and thin corpus callosum, periventricular frontal delayed myelination | Poor eye contact, no movement disorder | Hypotonia | Severe global developmental delay, motor skills: none | Not available | ASM (the names of the medicines were not included in the article) and ketogenic diet resistance |
| 11 Mirzaa, G.M. et al., 2013 [8] | Cerebral and cerebellar atrophy (postnatal microcephaly) | Cortical visual impairment, without movement disorder | Mixed (limb spasticity axial hypotonia) | Severe global developmental delay | Sleep apnea, facial dysmorphism, hypoplastic scrotum, G-tube | Difficult to treat, ketogenic diet and ASM resistance (the names of the medicines were not included in the article), ACTH resistance |
| Subject | MRI | Eye Contact, Movement Disorders | Tone/Reflexes | Motor Skills | Comorbidities | Treatment |
| 12 Mirzaa, G.M. et al., 2013 [8] | Cerebral and cerebellar atrophy | Cortical visual impairment, with movement disorder | Mixed (limb spasticity, axial hypotonia) | Severe global developmental delay | Gastroesophageal reflux, aspiration pneumonia, growth failure | Difficult to treat, ketogenic diet and ASM resistance (refractory to 17 antiepileptics—the names of the medicaments were not included in the article), responded briefly to treatment with ACTH |
| 13 Mirzaa, G.M. et al., 2013 [8] | Without cerebral atrophy | Cortical visual impairment, with movement disorder | Hypotonia | Severe global developmental delay | Dysphagia, aspiration pneumonia | Difficult to treat, ASM resistance (clorazepate, vigabatrin, levetiracetam, lamotrigine, oxcarbazepine, topiramate), the most effective therapy was topiramate, ACTH resistance, ketogenic diet with good response |
| 14 Mirzaa, G.M. et al., 2013 [8] | Without cerebral atrophy | Cortical visual impairment, with movement disorder | Severe hypotonia | Severe global developmental delay | Neurogenic bladder, G-tube, constipation | Difficult to treat, ASM resistance (the names of the medicines were not included in the article) |
| 15 Mirzaa, G.M. et al., 2013 [8] | Cerebral atrophy | Cortical visual impairment, without movement disorder | Severe hypotonia | Severe global developmental delay | G-tube, osteopenia, respiratory insufficiency | Difficult to treat, ASM resistance (the names of the medicines were not included in the article) |
| Subject | MRI | Eye Contact, Movement Disorders | Tone/Reflexes | Motor Skills | Comorbidities | Treatment |
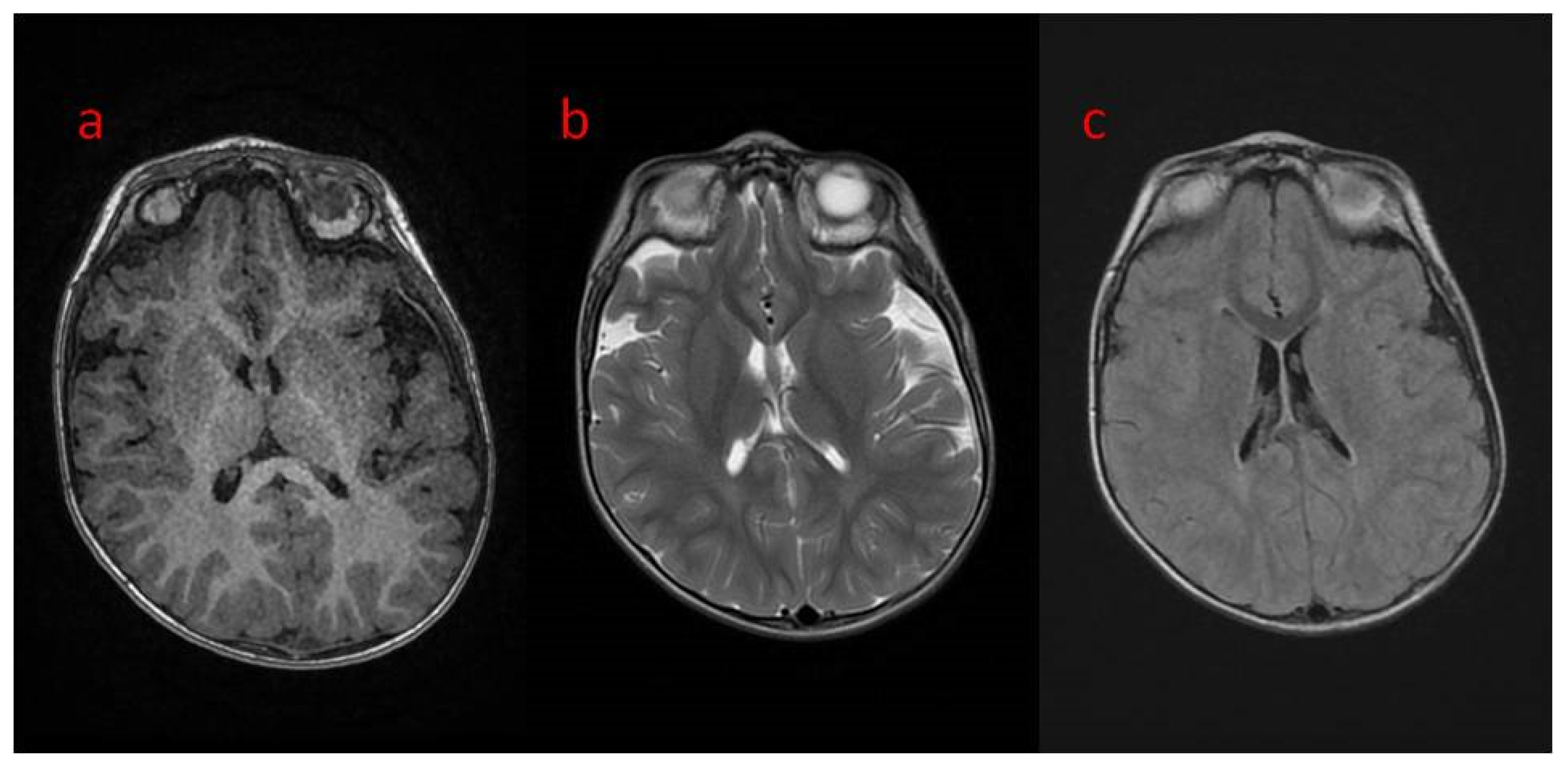
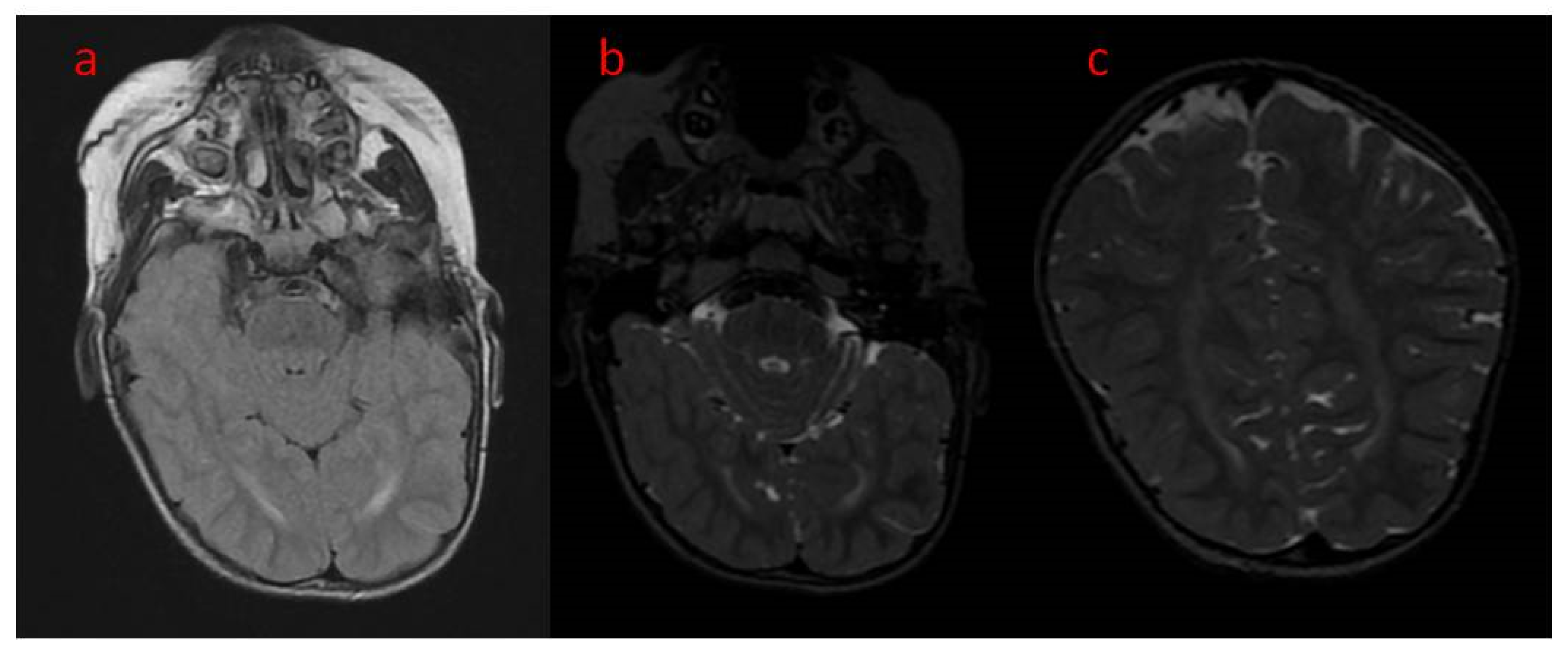
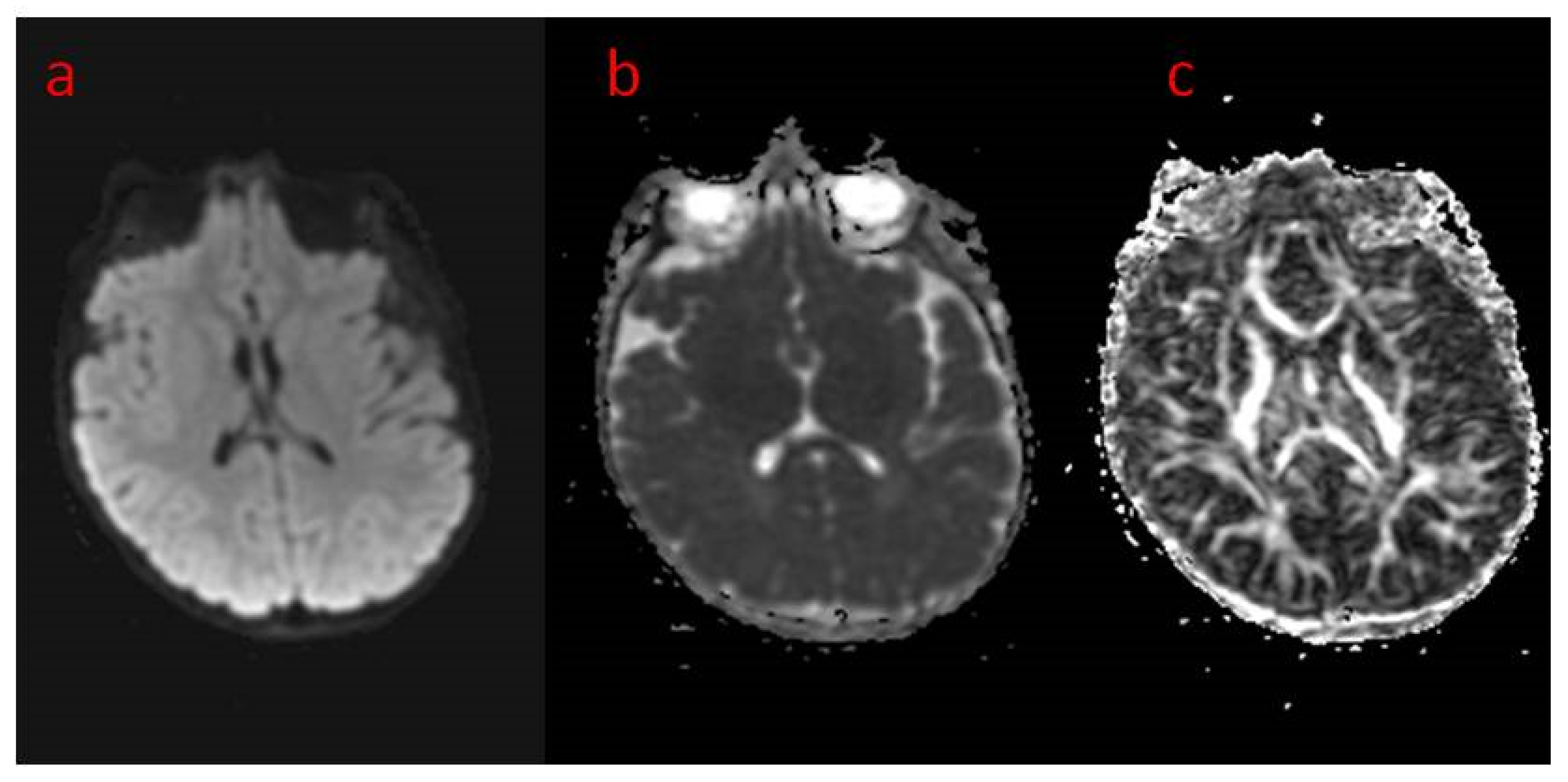
| 16 The present study | Areas of incomplete myelination (in the area of the triangles of the lateral ventricles and around the occipital horns) | Cortical visual impairment, poor eye contact, with movement disorder | Hypotonia, joint contractures | Severe global developmental delay | Dysphagia (only liquid food) | Difficult to treat, ASM resistance (valproic acid, topiramate, vigabatrin, phenobarbital, levetiracetam, phenytoin, clobazam, clonazepam, nitrazepam, lamotrigine, carbamazepine, and zonisamide), steroid therapy (adrenocorticotropic hormone, methylprednisolone—break), vagal nerve stimulation, ketogenic diet intolerance (due to reluctance to drink) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodak, M.; Jonderko, M.; Rozwadowska, P.; Machnikowska-Sokołowska, M.; Paprocka, J. CDKL5 Deficiency Disorder (CDD)—Rare Presentation in Male. Children 2022, 9, 1806. https://doi.org/10.3390/children9121806
Rodak M, Jonderko M, Rozwadowska P, Machnikowska-Sokołowska M, Paprocka J. CDKL5 Deficiency Disorder (CDD)—Rare Presentation in Male. Children. 2022; 9(12):1806. https://doi.org/10.3390/children9121806
Chicago/Turabian StyleRodak, Małgorzata, Mariola Jonderko, Patrycja Rozwadowska, Magdalena Machnikowska-Sokołowska, and Justyna Paprocka. 2022. "CDKL5 Deficiency Disorder (CDD)—Rare Presentation in Male" Children 9, no. 12: 1806. https://doi.org/10.3390/children9121806
APA StyleRodak, M., Jonderko, M., Rozwadowska, P., Machnikowska-Sokołowska, M., & Paprocka, J. (2022). CDKL5 Deficiency Disorder (CDD)—Rare Presentation in Male. Children, 9(12), 1806. https://doi.org/10.3390/children9121806







