Full Body Surface Coverage with Water-Equivalent Bolus as Novel Technique for Total Body Irradiation before Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoid Leukemia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pui, C.H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Radu, L.E.; Colita, A.; Pasca, S.; Tomuleasa, C.; Popa, C.; Serban, C.; Gheorghe, A.; Serbanica, A.; Jercan, C.; Marcu, A.; et al. Day 15 and day 33 minimal residual disease assessment for acute lymphoblastic leukemia patients treated according to the BFM ALL IC 2009 protocol: Single-center experience of 133 cases. Front. Oncol. 2020, 10, 923–931. [Google Scholar] [CrossRef] [PubMed]
- McNeer, J.L.; Devidas, M.; Dai, Y.; Carroll, A.J.; Heerema, N.A.; Gastier-Foster, J.M.; Kahwash, S.B.; Borowitz, M.J.; Wood, B.L.; Larsen, E.; et al. Hematopoietic stem-cell transplantation does not improve the poor outcome of children with hypodiploid acute lymphoblastic leukemia: A report from Children’s Oncology Group. J. Clin. Oncol. 2019, 37, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Rassiah, P.; Esiashvili, N.; Olch, A.J.; Hua, C.H.; Ulin, K.; Molineu, A.; Marcus, K.; Gopalakrishnan, M.; Pillai, S.; Kovalchuk, N.; et al. Practice patterns of pediatric total body irradiation techniques: A children’s oncology group survey. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, B.A.; Wong, J.Y.; Fog, L.S.; Losert, C.; Filippi, A.R.; Bentzen, S.M.; Balduzzi, A.; Specht, L. Total Body Irradiation in Haematopoietic Stem Cell Transplantation for Paediatric Acute Lymphoblastic Leukaemia: Review of the Literature and Future Directions. Front. Pediatr. 2021, 9, 774348–774376. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, C.R.; Horwitz, M.E.; Chino, J.P.; Craciunescu, O.; Steffey, B.; Folz, R.J.; Chao, N.J.; Rizzieri, D.A.; Marks, L.B. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: An assessment of risk factors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total body irradiation or chemotherapy conditioning in childhood ALL: A multinational, randomized, noninferiority phase III study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Yaray, K.; Damulira, E. Evaluation of volumetric modulated arc therapy (VMAT)—Based total body irradiation (TBI) in pediatric patients. Rep. Pract. Oncol. Radiother. 2021, 26, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A. Skin immunity. Arch. Immunol. Ther. Exp. 2018, 66, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Hui, S.; Hua, C.H.; Dusenbery, K.; Rassiah, P.; Kalapurakal, J.; Constine, L.; Esiashvili, N. Pulmonary toxicity after total body irradiation–critical review of the literature and recommendations for toxicity reporting. Front. Oncol. 2021, 11, 708906–708919. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Khaddour, K.; Hana, C.K.; Mewawalla, P. Hematopoietic stem cell transplantation. In StatPearls; Internet; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ronchese, F.; Hilligan, K.L.; Mayer, J.U. Dendritic cells and the skin environment. Curr. Opin. Immunol. 2020, 64, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Dutertre, C.A.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218, e20202345. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Velten, E.R.; Parsons, D.; Lee, P.; Chambers, E.; Abdulrahman, R.; Desai, N.B.; Dan, T.; Wardak, Z.; Timmerman, R.; Vusirikala, M.; et al. Volumetric modulated arc therapy enabled total body irradiation (VMAT-TBI): Six-year clinical experience and treatment outcomes. Transplant. Cell. Ther. 2022, 28, e1–e113. [Google Scholar] [CrossRef] [PubMed]
- Loginova, A.A.; Tovmasian, D.A.; Lisovskaya, A.O.; Kobyzeva, D.A.; Maschan, M.A.; Chernyaev, A.P.; Egorov, O.B.; Nechesnyuk, A.V. Optimized Conformal Total Body Irradiation methods with Helical TomoTherapy and Elekta VMAT: Implementation, Imaging, Planning and Dose Delivery for Pediatric Patients. Front. Oncol. 2022, 12, 785917–785930. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.; Hui, C.; Simiele, E.; Blomain, E.; Oh, J.; Bertaina, A.; Klein, O.; Shyr, D.; Jiang, A.; Hoppe, R.T.; et al. Volumetric modulated arc therapy total body irradiation in pediatric and adolescent/young adult patients undergoing stem cell transplantation: Early outcomes and toxicities. Pediatr. Blood Cancer. 2022, 69, e29689. [Google Scholar] [CrossRef] [PubMed]


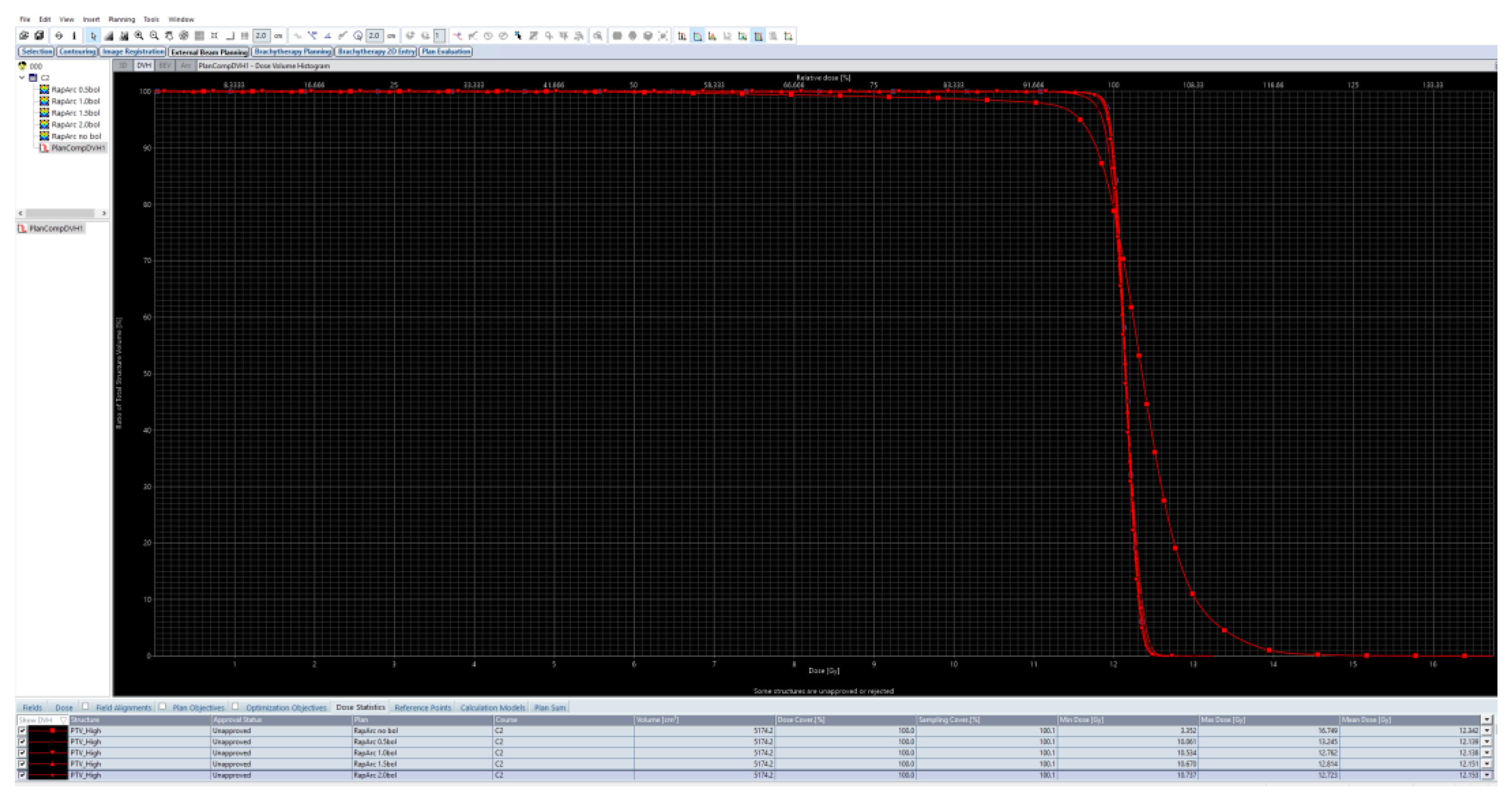
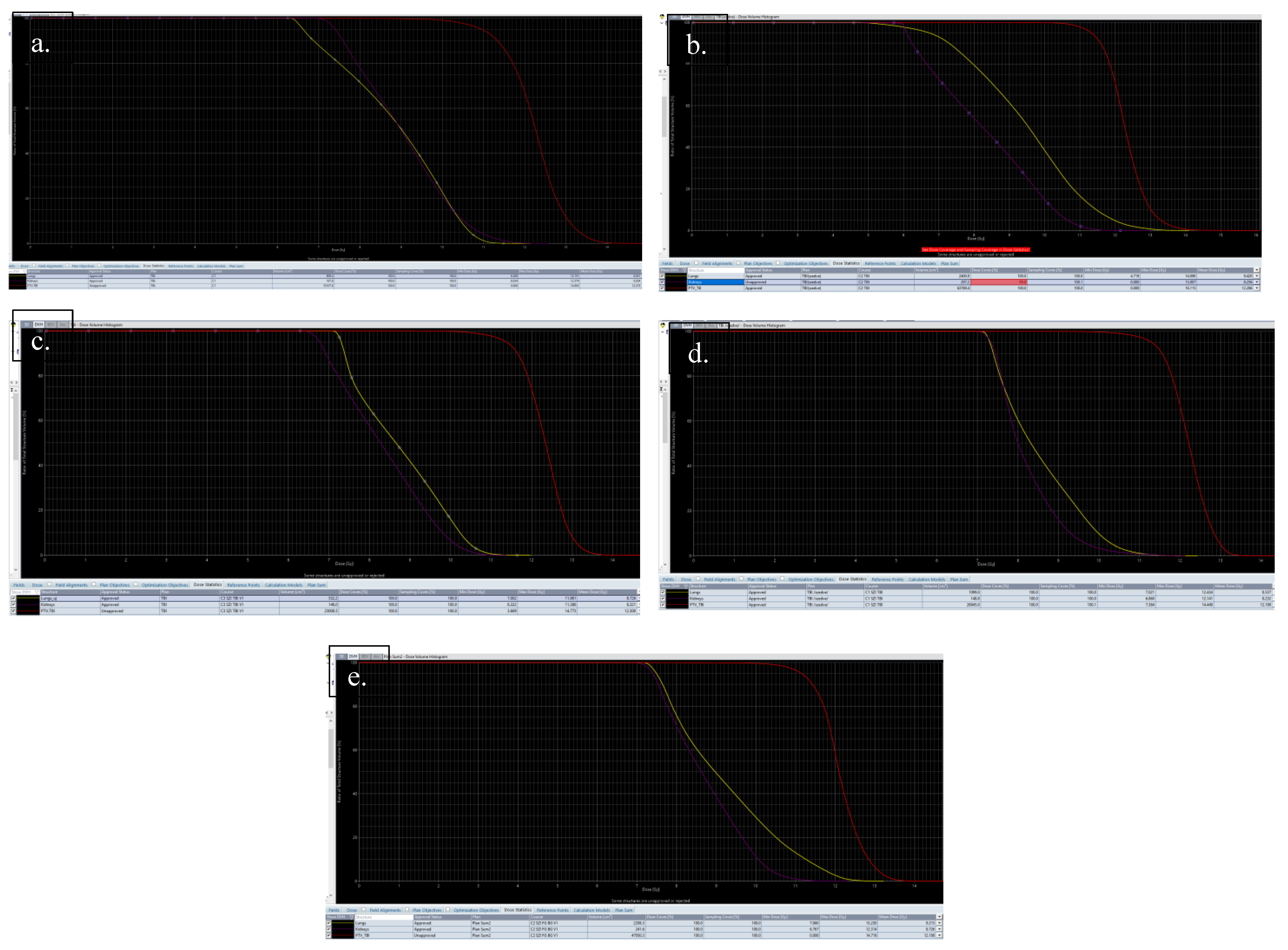
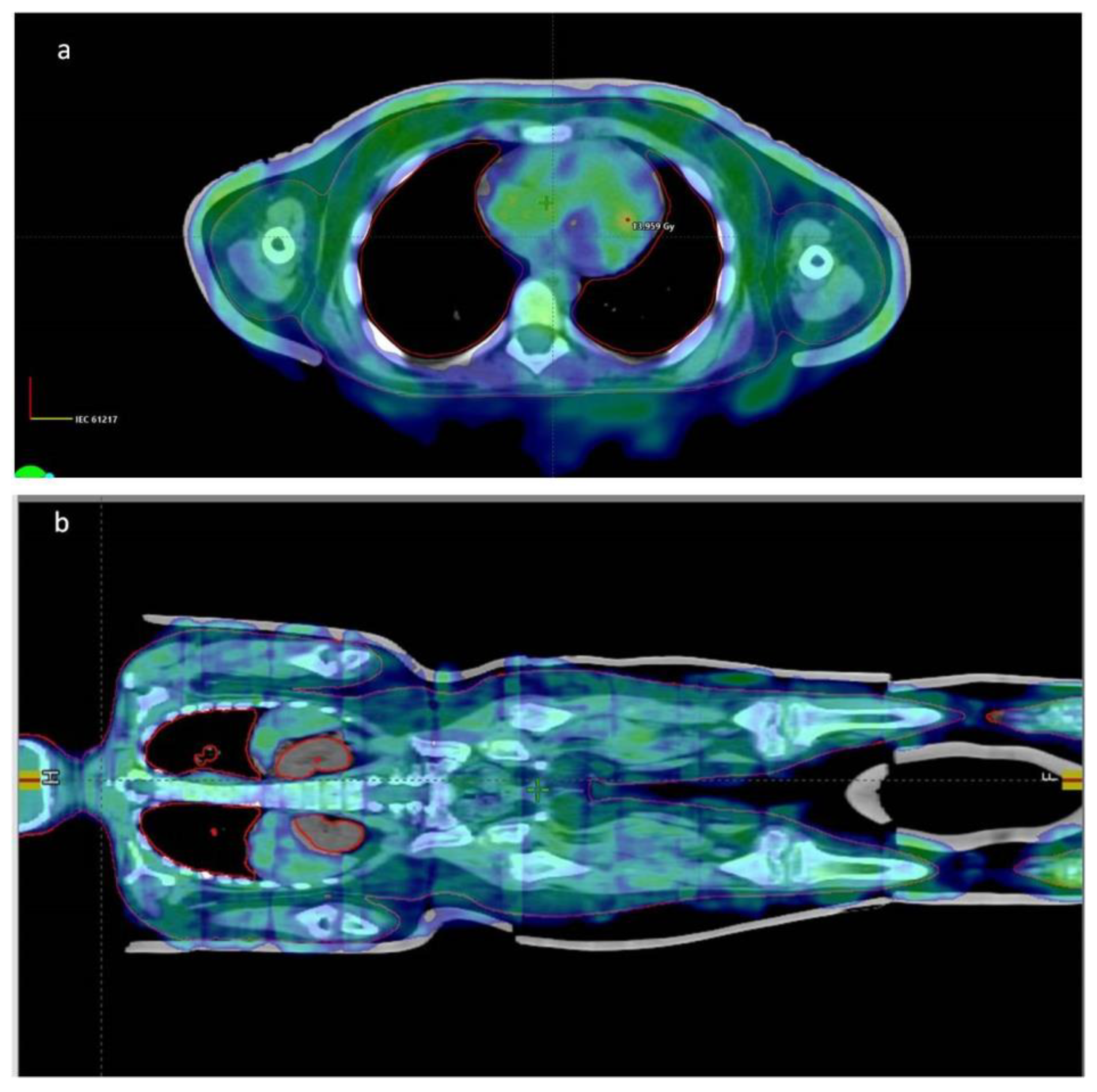
| Data of the Treated Patients | Patient-1 | Patient-2 | Patient-3 | Patient-4 | Patient-5 |
|---|---|---|---|---|---|
| Indication | pre-B ALL 2nd relapse, ETV6-RUNX1 (t12;21), CNS 1 | T-ALL HR, STIL-TAL1 fusion, CNS 3 | B-ALL early relapse, chr 21 tetrasomy, RUNX1 signal overexpression, CNS 1 | CML with 2 ALL blastic phase, bcr/abl positive, CNS 1 | pre-B-cell ALL relapse bcr/abl positive, CNS 1 |
| Last previous treatment | ALL-IC REL 2016-HR + blinatumomab | ALL-IC BFM 2009 | ALL-IC REL 2016 + blinatumomab | ALL-IC REL 2016 + dasatinib, blinatumomab | ALL-IC REL 2016 + dasatinib |
| Number of administered CD34 positive (cells /kg) | 9.2 × 106 | 0.8 × 106 | 3.64 × 106 | 8 × 106 | 5 × 106 |
| Donor type (HLA) | Matched unrelated donor (9/10) | Matched family donor (10/10) | Matched unrelated donor (12/12) | Matched unrelated donor (11/12) | Matched unrelated donor (10/12) |
| Height of pts (cm) | 134 | 178 | 108 | 131 | 158 |
| Body weight of pts (kg) | 36.4 | 67 | 24 | 27.7 | 53 |
| Age at transplant of pts (yrs) | 10.02 | 16.52 | 5.45 | 8.75 | 17.39 |
| Gender | Female | Male | Male | Male | Male |
| GVHD prophylaxis | CSA, MTX | CSA, MTX | CSA, MTX | CSA, MTX | CSA, MTX |
| Time to neutrophil engraftment | Day + 19 | Day + 18 | Day + 27 | Day + 23 | Day + 21 |
| Adverse events | Perianal abscess (Gr3), mucositis (Gr3) sepsis (Gr3) | Mucositis (Gr3), sepsis (Gr2) | Mucositis (Gr2), sepsis (Gr2), Adenovirus viraemia | Secondary graft failure *, Adenovirus and CMV viraemia | Seizure (Gr3), mucositis (Gr4), bowel acute GVHD (Gr4), BKV and Adenovirus related (Gr2) haemorrhagic cystitis and viremia |
| Conditioning regimen | ALL PED SCT Forum (TBI, etoposide) | ALL PED SCT Forum (TBI, etoposide) | ALL PED SCT Forum (TBI, etoposide) | ALL PED SCT Forum (TBI, etoposide) | ALL PED SCT Forum (TBI, etoposide) |
| Follow-up time (yrs) | 2.11 | 0.69 | 1.46 | 1.43 | 0.43 |
| Data of the Radiotherapy Plans | |||||
|---|---|---|---|---|---|
| Time min (min) | 43 | 49 | 39 | 39 | 51 |
| Time max (min) | 125 | 58 | 49 | 44 | 61 |
| Time avg (min) | 60.16 | 53.17 | 43 | 40.33 | 53.66 |
| CBCT numbers | 18 | 18 | 18 | 18 | 18 |
| Kidneys mean dose (Gy) | 9.034 | 8.256 | 8.321 | 8.232 | 8.726 |
| Lungs mean dose (Gy) | 8.851 | 9.42 | 8.726 | 8.537 | 9.215 |
| Iso centers | 9 | 13 | 9 | 10 | 12 |
| Technic | VMAT | VMAT | VMAT | VMAT | VMAT/IMRT |
| Arcs/Fields | 18 | 24 | 18 | 20 | 14 Field IMRT + 19 |
| Sum MU | 2655.4 | 4987.1 | 4449 | 4094.5 | 6483.8 |
| Energy | 6x photon | 6x photon | 6x photon | 6x photon | 6x photon |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furka, A.; Nagy, Z.; Szabó, I.; Fekete, G.; Kelemen, Á.; Bolobás, G.; Sebők, G.; Molnár, T.; Árvai, J.; Tornyi, I.; et al. Full Body Surface Coverage with Water-Equivalent Bolus as Novel Technique for Total Body Irradiation before Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoid Leukemia. Children 2022, 9, 1740. https://doi.org/10.3390/children9111740
Furka A, Nagy Z, Szabó I, Fekete G, Kelemen Á, Bolobás G, Sebők G, Molnár T, Árvai J, Tornyi I, et al. Full Body Surface Coverage with Water-Equivalent Bolus as Novel Technique for Total Body Irradiation before Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoid Leukemia. Children. 2022; 9(11):1740. https://doi.org/10.3390/children9111740
Chicago/Turabian StyleFurka, Andrea, Zsofia Nagy, Imre Szabó, Gábor Fekete, Ágnes Kelemen, Gábor Bolobás, Gábriel Sebők, Tünde Molnár, János Árvai, Ilona Tornyi, and et al. 2022. "Full Body Surface Coverage with Water-Equivalent Bolus as Novel Technique for Total Body Irradiation before Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoid Leukemia" Children 9, no. 11: 1740. https://doi.org/10.3390/children9111740
APA StyleFurka, A., Nagy, Z., Szabó, I., Fekete, G., Kelemen, Á., Bolobás, G., Sebők, G., Molnár, T., Árvai, J., Tornyi, I., Kostyál, L., Révész, J., & Hauser, P. (2022). Full Body Surface Coverage with Water-Equivalent Bolus as Novel Technique for Total Body Irradiation before Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoid Leukemia. Children, 9(11), 1740. https://doi.org/10.3390/children9111740







