Timing of Systemic Steroids and Neurodevelopmental Outcomes in Infants < 29 Weeks Gestation
Abstract
1. Introduction
2. Methods
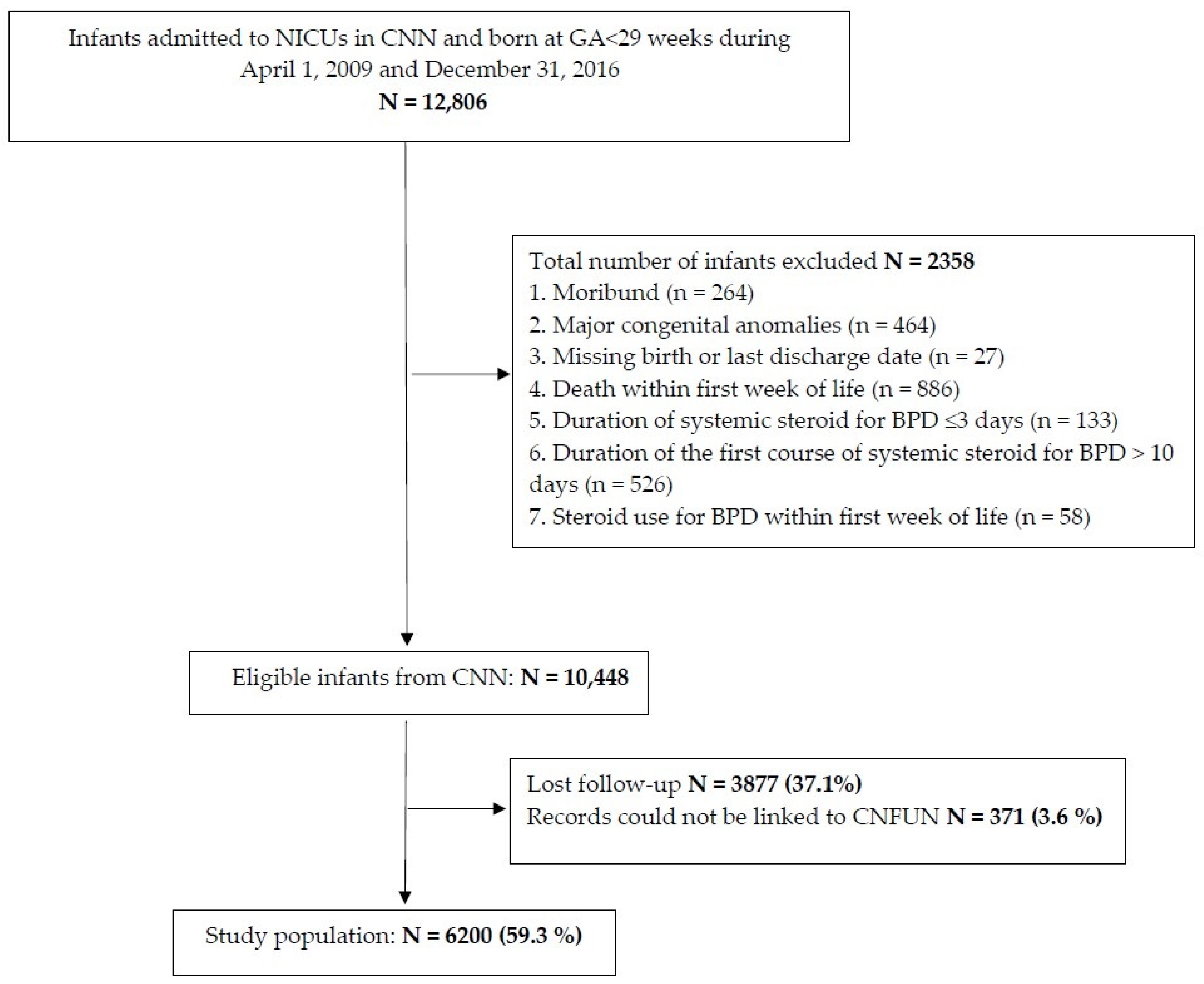
2.1. Study Design and Population
2.2. Outcomes
2.3. Data Extraction and Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
Appendix A.1. Canadian Neonatal Network Site Investigators
Appendix A.2. Canadian Neonatal Follow-Up Network Site Investigators and Steering Committee
References
- Lui, K.; Lee, S.K.; Kusuda, S.; Adams, M.; Vento, M.; Reichman, B.; Darlow, B.A.; Lehtonen, L.; Modi, N.; Norman, M.; et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J. Pediatr. 2019, 215, 32–40.e14. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.S.; Sankaran, K.; Aziz, K.; Allen, A.C.; Seshia, M.; Ohlsson, A.; Lee, S.K.; Canadian Neonatal Network. Outcomes of preterm infants < 29 weeks gestation over 10-year period in Canada: A cause for concern? J. Perinatol. 2012, 32, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.S.; Chan, T.; Lands, L.; Menzies, D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can. Respir. J. 2011, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Lo, J.W.; Marlow, N.; Calvert, S.A.; Greenough, A.; Peacock, J.L. Postnatal dexamethasone, respiratory and neurodevelopmental outcomes at two years in babies born extremely preterm. PLoS ONE 2017, 12, e0181176. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Nanda, D.; Bandiya, P.; Ahmed, J.; Garg, A.; Roehr, C.C.; Nangia, S. Assessment of Postnatal Corticosteroids for the Prevention of Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Network Meta-analysis. JAMA Pediatr. 2021, 175, e206826. [Google Scholar] [CrossRef]
- Lemyre, B.; Dunn, M.; Thebaud, B. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia in preterm infants. Paediatr. Child Health 2020, 25, 322–331. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Kreider, M.L.; Tate, C.A.; Cousins, M.M.; Oliver, C.A.; Seidler, F.J.; Slotkin, T.A. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: Critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology 2006, 31, 12–35. [Google Scholar] [CrossRef]
- McGowan, J.E.; Sysyn, G.; Petersson, K.H.; Sadowska, G.B.; Mishra, O.P.; Delivoria-Papadopoulos, M.; Stonestreet, B.S. Effect of dexamethasone treatment on maturational changes in the NMDA receptor in sheep brain. J. Neurosci. 2000, 20, 7424–7429. [Google Scholar] [CrossRef]
- Malaeb, S.N.; Stonestreet, B.S. Steroids and injury to the developing brain: Net harm or net benefit? Clin. Perinatol. 2014, 41, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: A systematic review of RCTs. BMC Pediatr. 2001, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N. Engl. J. Med. 2004, 350, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Kothadia, J.M.; Klinepeter, K.L.; Goldstein, D.J.; Jackson, B.G.; Weaver, R.G.; Dillard, R.G. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: Outcome of study participants at 1-year adjusted age. Pediatrics 1999, 104 (Pt 1), 15–21. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef]
- Wilson-Costello, D.; Walsh, M.C.; Langer, J.C.; Guillet, R.; Laptook, A.R.; Stoll, B.J.; Shankaran, S.; Finer, N.N.; Van Meurs, K.P.; Engle, W.A.; et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: Effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics 2009, 123, e430–e437. [Google Scholar] [CrossRef]
- Harmon, H.M.; Jensen, E.A.; Tan, S.; Chaudhary, A.S.; Slaughter, J.L.; Bell, E.F.; Wyckoff, M.H.; Hensman, A.M.; Sokol, G.M.; DeMauro, S.B.; et al. Timing of postnatal steroids for bronchopulmonary dysplasia: Association with pulmonary and neurodevelopmental outcomes. J. Perinatol. 2020, 40, 616–627. [Google Scholar] [CrossRef]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B. DART Study Investigators. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef]
- Canadian Neonatal Network Abstractor’s Manual v.3.5.2 (Released February 2022); CNN: Toronto, ON, Canada; Available online: http://www.canadianneonatalnetwork.org/portal/Portals/0/CNN%20Manuals/CNN%20Manual_20220201.pdf (accessed on 1 October 2021).
- Shah, P.S.; Seidlitz, W.; Chan, P.; Yeh, S.; Musrap, N.; Lee, S.K.; Shah, P.S.; Data abstractors of the Canadian Neonatal Network. Internal Audit of the Canadian Neonatal Network Data Collection System. Am. J. Perinatol. 2017, 34, 1241–1249. [Google Scholar] [CrossRef]
- de Vries, L.S.; Eken, P.; Dubowitz, L.M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 1992, 49, 1–6. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Walsh, M.C. Neonatal necrotizing enterocolitis: Pathogenesis, classification, and spectrum of illness. Curr. Probl. Pediatr. 1987, 17, 213–288. [Google Scholar] [CrossRef]
- Synnes, A.; Luu, T.M.; Moddemann, D.; Church, P.; Lee, D.; Vincer, M.; Ballantyne, M.; Majnemer, A.; Creighton, D.; Yang, J.; et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F234–F235. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.L.; Palisano, R.J.; Bartlett, D.J.; Galuppi, B.E.; Russell, D.J. Development of the Gross Motor Function Classification System for Cerebral Palsy. Dev. Med. Child Neurol. 2008, 50, 249–253. [Google Scholar] [CrossRef]
- Chang, K.-H.; Yeh, C.-M.; Yeh, C.-Y.; Huang, C.-C.; Hsu, K.-S. Neonatal dexamethasone treatment exacerbates hypoxic-ischemic brain injury. Mol. Brain 2013, 6, 18. [Google Scholar] [CrossRef]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchée, A.; Bolot, P.; et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef]
| Variables * | No sPNS Exposure (N = 5137) | sPNS Exposure (N = 1063) | p-Value |
|---|---|---|---|
| Maternal age, mean (SD) | 31.2 (5.82) | 31.3 (5.71) | 0.7 |
| Maternal hypertension, % (n/N) | 1.9 (800/5008) | 17.5 (184/1050) | 0.22 |
| Maternal diabetes, % (n/N) | 9.6 (472/4904) | 7.8 (79/1014) | 0.07 |
| Antenatal steroid use, % (n/N) | 89.2 (4486/5030) | 91.6 (958/1046) | 0.02 |
| Cesarean delivery, % (n/N) | 58 (2971/5120) | 58.3 (618/1061) | 0.89 |
| Singleton, % (n/N) | 72.5 (3722/5135) | 74.4 (791/1063) | 0.2 |
| Gestational age at birth (weeks), median (IQR) | 27 (25, 28) | 25 (24, 26) | <0.001 |
| Gestational age group, % (n/N) 22–25 (weeks) 26–28 (weeks) | 25.8 (1323/5137) 74.2 (3814/5137) | 56 (595/1063) 44 (468/1063) | <0.001 |
| Birth weight (g), mean (SD) | 950 (234) | 799 (202) | <0.001 |
| Sex (male), % (n/N) | 53.4 (2742/5132) | 55.6 (589/1060) | 0.2 |
| Small gestational age, % (n/N) | 8 (411/5134) | 10.7 (113/1061) | 0.005 |
| Apgar score < 7 at 5 min, % (n/N) | 38.1 (1938/5087) | 45.1 (474/1052) | <0.001 |
| SNAP-II score > 20, % (n/N) | 26.5 (1354/5109) | 36.8 (390/1061) | <0.001 |
| Severe neurological injury (≥grade III IVH or PVL), % (n/N) | 12.9 (646/5008) | 15.9 (166/1038) | 0.008 |
| PDA, % (n/N) | 55.1 (2815/5106) | 71.9 (759/1055) | 0.001 |
| Variables * | No sPNS Exposure (N = 5137) | sPNS Exposure (N = 1063) | p-Value |
|---|---|---|---|
| BPD, % (n/N) | 44 (1968/4472) | 63.4 (637/1004) | <0.001 |
| Severe ROP (≥stage III or requiring therapy), % (n/N) | 9 (333/3483) | 24 (236/978) | <0.001 |
| NEC stage ≥ 2, % (n/N) | 9.6 (493/5127) | 8 (87/1062) | 0.15 |
| SIP, % (n/N) | 3 (138/4568) | 4 (37/964) | 0.19 |
| Late-onset sepsis, %(n/N) | 24.6 (1263/5137) | 34.6 (368/1063) | <0.001 |
| Mortality before discharge from NICUs, %(n/N) | 13.1 (674/5137) | 9 (92/1063) | <0.001 |
| Duration of NICU stay (days), median (IQR) | 64 (36, 91) | 104 (65, 128) | <0.001 |
| Variables * | Postnatal Age at Exposure to the First sPNS (for BPD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 2 N = 85 | Week 3 N = 186 | Week 4 N = 245 | Week 5 N = 199 | Week 6 N = 134 | Week 7 N = 88 | Week 8–9 N = 62 | ≥Week 10 N = 64 | |
| Maternal hypertension, % (n/N) | 12 (10/84) | 19 (34/184) | 17 (42/241) | 16 (32/199) | 16 (21/130) | 16 (14/87) | 14 (9/62) | 35 (22/63) |
| Antenatal steroid use, % (n/N) | 84 (71/84) | 92 (165/180) | 93 (222/239) | 93 (185/199) | 92 (121/132) | 84 (72/86) | 98 (61/62) | 95 (61/64) |
| Cesarean delivery % (n/N) | 67 (57/85) | 57 (106/186) | 59 (145/244) | 56 (111/199) | 56 (75/133) | 57 (50/88) | 56 (35/62) | 61 (39/64) |
| Singleton, % (n/N) | 80 (68/85) | 79 (147/186) | 71 (174/245) | 76.4 (152/199) | 72.4 (97/134) | 70.5 (62/88) | 64.5 (40/62) | 79.7 (51/64) |
| Gestational age at birth (weeks) median (IQR) | 25 (24, 26) | 25 (24, 26) | 25 (24, 26) | 25 (25, 26) | 25 (24, 26) | 25 (24, 26) | 26 (24, 27) | 26 (25, 27) |
| Gestational age group, % (n/N) 22–25 (weeks) 26–28 (weeks) | 68 (58/85) 32 (27/85) | 57 (106/186) 43 (80/186) | 57 (139/245) 43 (106/245) | 55 (110/199) 45 (89/199) | 54 (72/134) 46 (62/134) | 57 (50/88) 43 (38/88) | 47 (29/62) 53 (33/62) | 48 (31/64) 52 (33/64) |
| Birth Weight (g), mean (SD) | 780 (187.18) | 814 (184.43) | 802 (187.59) | 786 (222.47) | 822 (185.21) | 791 (262.86) | 819 (211.99) | 756 (176.8) |
| Sex (male), % (n/N) | 65 (55/85) | 55 (103/186) | 58 (142/243) | 52 (103/199) | 54 (73/134) | 51 (44/87) | 55 (34/62) | 55 (35/64) |
| Small gestational age, % (n/N) | 11 (9/85) | 7 (13/186) | 9 (23/243) | 13 (25/199) | 9 (12/134) | 12 (11/88) | 8 (5/62) | 23 (15/64) |
| Apgar score < 7 at 5 min, % (n/N) | 52 (44/84) | 48 (89/184) | 40 (97/243) | 41 (82/199) | 47 (61/131) | 38 (33/86) | 56 (35/62) | 52 (33/63) |
| SNAP-II score > 20, % (n/N) | 43 (36/84) | 29 (54/186) | 42 (102/244) | 34 (67/199) | 33 (44/134) | 39 (34/88) | 44 (27/62) | 41 (26/64) |
| Outcomes * | Age at First sPNS for BPD | |||||||
|---|---|---|---|---|---|---|---|---|
| Week 2 N = 85 | Week 3 N =186 | Week 4 N = 245 | Week 5 N = 199 | Week 6 N = 134 | Week 7 N = 88 | Week 8–9 N = 62 | ≥Week 10 N = 64 | |
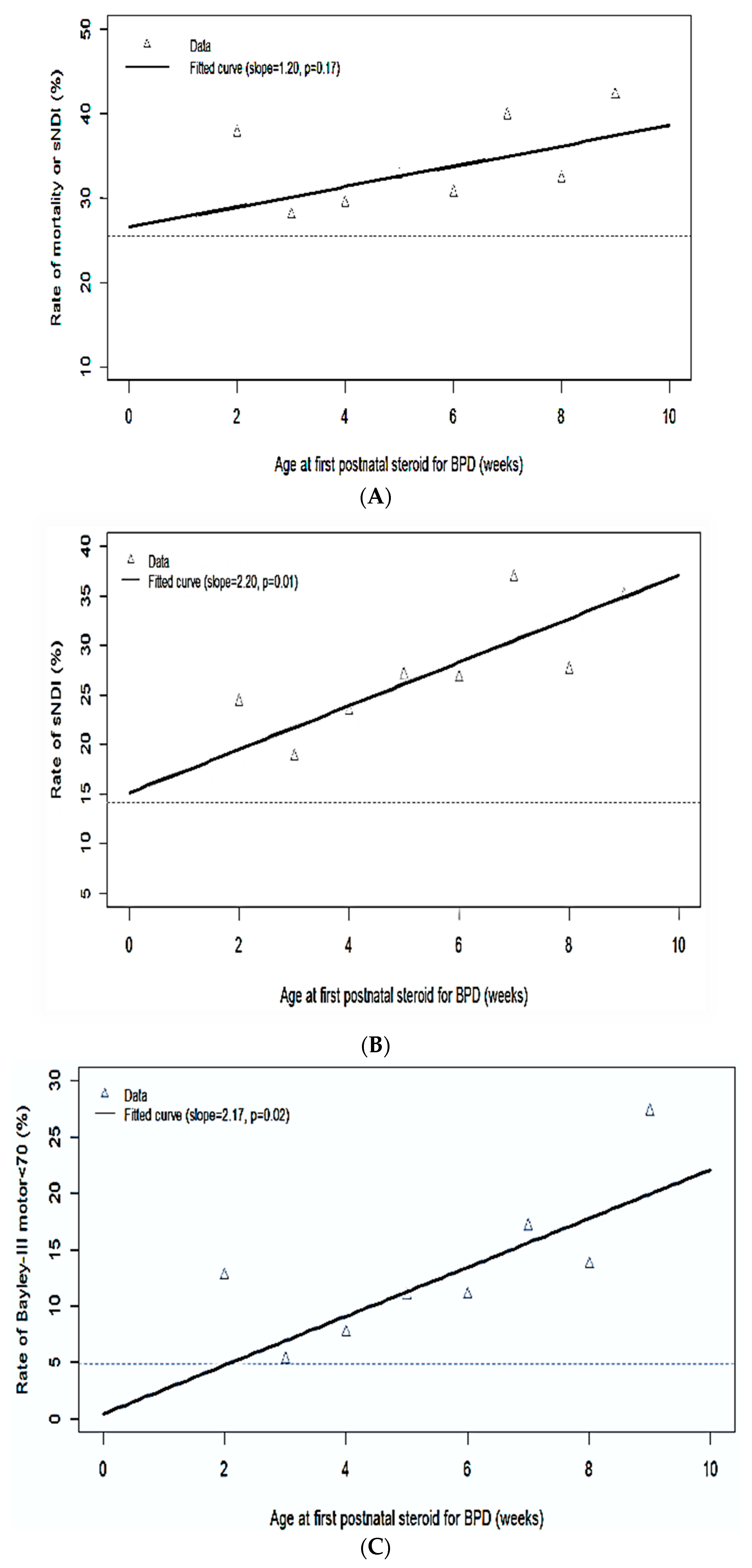
| Mortality or sNDI, % (n/N) | 38 (32/85) | 28 (52/186) | 29 (72/245) | 33 (65/199) | 31 (41/134) | 40 (35/88) | 32 (20/62) | 42 (27/64) |
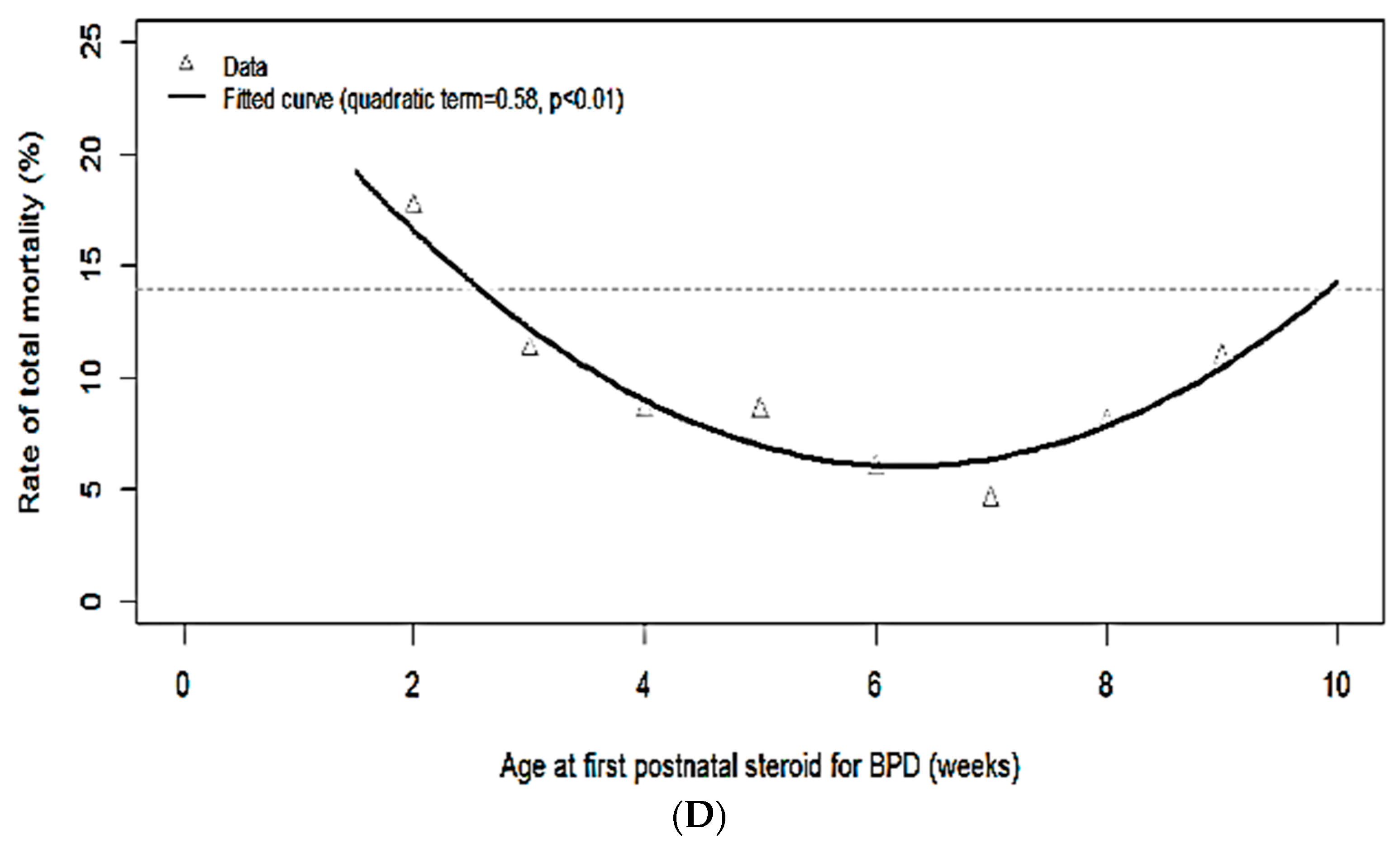
| Mortality, % (n/N) | 18 (15/85) | 11 (21/186) | 9 (21/245) | 9 (17/199) | 6 (8/134) | 5 (4/88) | 8 (5/62) | 11 (7/64) |
| sNDI, % (n/N) | 24 (17/70) | 19 (31/165) | 24 (53/226) | 27 (50/185) | 27 (34/127) | 37 (31/84) | 28 (16/58) | 35 (20/57) |
| NDI, % (n/N) | 60 (42/70) | 55 (90/165) | 53 (119/226) | 52 (96/185) | 55 (70/127) | 61 (51/84) | 62 (36/58) | 61 (35/57) |
| Any CP (GMFCS ≥ 1), % (n/N) | 9 (6/67) | 8 (12/161) | 7 (16/221) | 8 (15/179) | 9 (11/124) | 11 (9/83) | 9 (5/55) | 13 (7/55) |
| Cognitive composite < 85, % (n/N) | 25 (16/65) | 21 (33/155) | 20 (41/205) | 19 (33/175) | 21 (25/120) | 32 (24/76) | 25 (13/53) | 35 (16/46) |
| Cognitive composite < 70, % (n/N) | 9 (6/65) | 5 (8/155) | 4 (8/205) | 6 (10/175) | 7 (8/120) | 7 (5/76) | 8 (4/53) | 13 (6/46) |
| Language composite < 85, % (n/N) | 48 (31/64) | 45 (67/149) | 44 (87/200) | 40 (71/176) | 41 (48/117) | 49 (36/73) | 49 (24/49) | 58 (26/45) |
| Language composite < 70, % (n/N) | 22 (14/64) | 13 (19/149) | 17 (34/200) | 17 (30/176) | 17 (20/117) | 27 (20/73) | 20 (10/49) | 29 (13/45) |
| Motor composite < 85, % (n/N) | 35 (22/63) | 25 (38/152) | 25 (49/195) | 27 (47/173) | 33 (39/118) | 41 (31/76) | 31 (16/51) | 48 (21/44) |
| Motor composite < 70, % (n/N) | 13 (8/63) | 5 (8/152) | 8 (15/195) | 11 (19/173) | 11 (13/118) | 17 (13/76) | 14 (7/51) | 27 (12/44) |
| Outcomes * | Adjusted OR * (95% CI) (Per 1-Week Increase in the Postnatal Age) | p-Value |
|---|---|---|
| Mortality or sNDI | 1.094 (0.942, 1.024) | 0.22 |
| sNDI | 1.015 (1.004, 1.029) | 0.04 |
| NDI | 1.007 (0.995, 1.019) | 0.25 |
| CP (GMFCS ≥ 1) | 1.01 (0.99, 1.03) | 0.35 |
| Bayley III cognitive composite < 85 | 1.009 (0.999, 1.019) | 0.05 |
| Bayley III language composite < 85 | 1.006 (0.986 1.013) | 0.11 |
| Bayley III motor composite < 85 | 1.015 (0.999, 1.030) | 0.06 |
| Bayley III cognitive composite < 70 | 1.013 (0.995, 1.033) | 0.15 |
| Bayley III language composite < 70 | 1.012 (0.997, 1.027) | 0.11 |
| Bayley III motor composite < 70 | 1.026 (1.009, 1.044) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandraju, H.; Jasani, B.; Shah, P.S.; Church, P.T.; Luu, T.M.; Ye, X.Y.; Stavel, M.; Mukerji, A.; Shah, V.; the CNN Investigators; et al. Timing of Systemic Steroids and Neurodevelopmental Outcomes in Infants < 29 Weeks Gestation. Children 2022, 9, 1687. https://doi.org/10.3390/children9111687
Kandraju H, Jasani B, Shah PS, Church PT, Luu TM, Ye XY, Stavel M, Mukerji A, Shah V, the CNN Investigators, et al. Timing of Systemic Steroids and Neurodevelopmental Outcomes in Infants < 29 Weeks Gestation. Children. 2022; 9(11):1687. https://doi.org/10.3390/children9111687
Chicago/Turabian StyleKandraju, Hemasree, Bonny Jasani, Prakesh S. Shah, Paige T. Church, Thuy Mai Luu, Xiang Y. Ye, Miroslav Stavel, Amit Mukerji, Vibhuti Shah, the CNN Investigators, and et al. 2022. "Timing of Systemic Steroids and Neurodevelopmental Outcomes in Infants < 29 Weeks Gestation" Children 9, no. 11: 1687. https://doi.org/10.3390/children9111687
APA StyleKandraju, H., Jasani, B., Shah, P. S., Church, P. T., Luu, T. M., Ye, X. Y., Stavel, M., Mukerji, A., Shah, V., the CNN Investigators, & the CNFUN Investigators. (2022). Timing of Systemic Steroids and Neurodevelopmental Outcomes in Infants < 29 Weeks Gestation. Children, 9(11), 1687. https://doi.org/10.3390/children9111687






