Spontaneous Pneumomediastinum in a 16-Year-Old Patient with SARS-CoV-2 Infection: A North-East Romanian Case
Abstract
1. Introduction
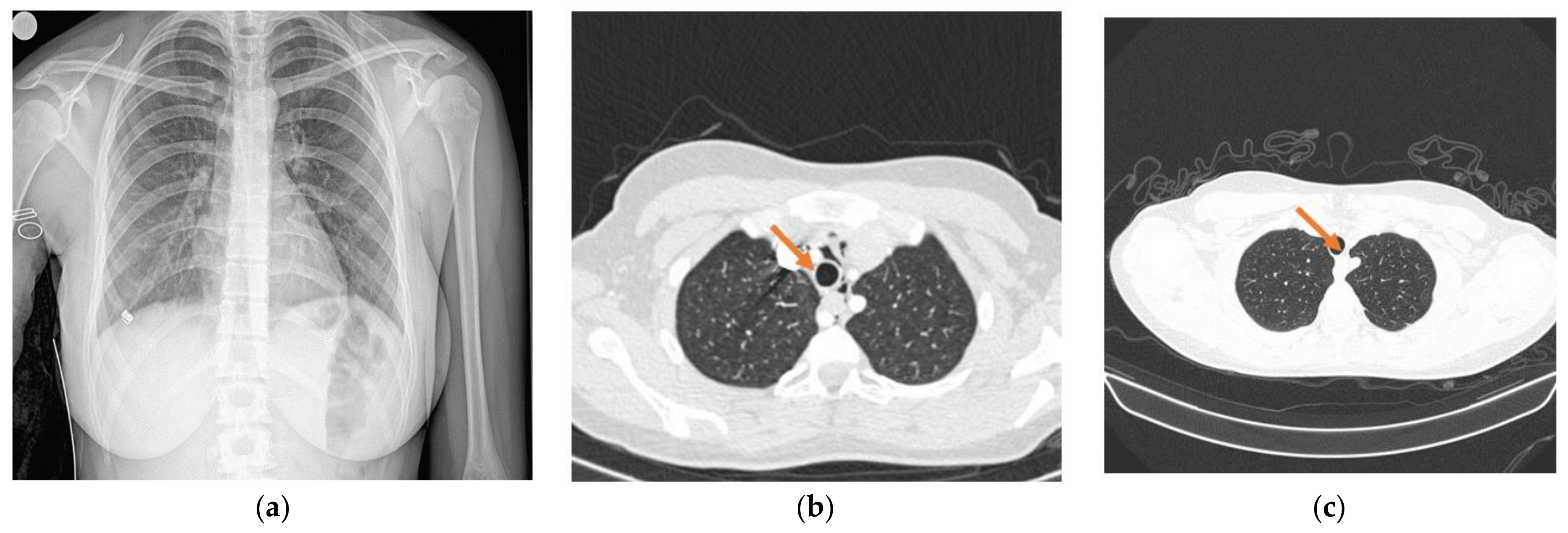
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Elhakim, T.S.; Abdul, H.S.; Romero, C.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumo-thorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. 2020, 13, e239489. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.G.; Quinn, N.; Byrne, C.; Kassab, G.; Whelan, S.; Colleran, G.C. Pneumomediastinum in a child with severe COVID-19. BJR Case Rep. 2020, 7, 20200062. [Google Scholar] [CrossRef] [PubMed]
- Mondello, B.; Pavia, R.; Ruggeri, P.; Barone, M.; Barresi, P.; Monaco, M. Spontaneous pneumomediastinum: Experience in 18 adult patients. Lung 2007, 185, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Murayama, S.; Gibo, M.; Akamine, T.; Nagata, O. Frequent cause of the Macklin effect in spontaneous pneumomediastinum: Demonstration by multidetector-row computed tomography. J. Comput. Assist. Tomogr. 2006, 30, 92–94. [Google Scholar] [CrossRef]
- Forshaw, M.J.; Khan, A.Z.; Strauss, D.C.; Botha, A.J.; Mason, R.C. Vomiting-induced pneumomediastinum and subcutaneous emphysema does not always indicate Boerhaave’s syndrome: Report of six cases. Surg. Today 2007, 37, 888–892. [Google Scholar] [CrossRef]
- Stack, A.M.; Caputo, G.L. Pneumomediastinum in childhood asthma. Pediatr. Emerg. Care 1996, 12, 98–101. [Google Scholar] [CrossRef]
- Yellin, A.; Gapanavicius, M.G.; Lieberman, Y. Spontaneous pneumomediastinum: Is it a rare cause of chest pain? Thorax 1983, 38, 383–385. [Google Scholar] [CrossRef]
- Jougon, J.B.; Ballester, M.; Delcambre, F.; Mac Bride, T.; Dromer, C.E.H.; Velly, J.F. Assessment of spontaneous pneumomediastinum: Experience with 12 patients. Ann. Thorac. Surg. 2003, 75, 1711–1714. [Google Scholar] [CrossRef]
- Lupieri, E.; Boffi, A.; Ltaief, Z.; Schneider, A.; Abed-Maillard, S.; Chiche, J.; Oddo, M.; Piquilloud, L. Response to the first awake prone positioning relates with intubation rate in SARS-CoV-2 patients suffering from acute respiratory failure with moderate to severe hypoxaemia: A retrospective study. Swiss Med. Wkly. 2022, 35, w30212. [Google Scholar]
- Johnson, K.B.; Carroll, V.G.; Parker, H.G. Pneumomediastinum after COVID-19. Glob. Pediatr. Health 2022, 31, 2333794X221101773. [Google Scholar] [CrossRef]
- Patel, N.; Nicolae, R.; Geropoulos, G.; Mandal, P.; Christou, C.D.; Gavala, M.; Madouros, N.; Papapanou, M.; Mogal, R.; Giannis, D.; et al. Pneumomediastinum in the COVID-19 era: To drain or not to drain? Monaldi Arch. Chest Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.M.L.; Gordo, M.L.P.; Tascón, A.D.; Vélez, S.O. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg. Radiol. 2020, 27, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Loffi, M.; Regazzoni, V.; Sergio, P.; Martinelli, E.; Stifani, I.; Quinzani, F.; Robba, D.; Cotugno, A.; DeDe, M.; Danzi, G.B. Spontaneous pneumomediastinum in COVID-19 pneumonia. Monaldi Arch. Chest Dis. 2020, 90, 604–607. [Google Scholar] [CrossRef]
- Dixit, A.; Uvaise, M.; Canet-Tarres, A.; Lillie, J. Spontaneous Massive Pneumomediastinum in a Previously Well Infant with COVID-19. Pediatrics 2021, 148, e2021051904. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, K.A.; Williams, A.E.; Langham, J.J.; Wu, L.; Krafty, R.T.; Furtado, A.D.; Zuckerbraun, N.S.; Manole, M.D. Management and Outcomes of Spontaneous Pneumomediastinum in Children. Pediatr. Emerg. Care 2021, 37, E1051–E1056. [Google Scholar] [CrossRef]
- Chalumeau, M.; le Clainche, L.; Sayeg, N.; Sannier, N.; Michel, J.L.; Marianowski, R.; Jouvet, P.; Scheinmann, P.; de Blic, J. Spontaneous pneumomediastinum in children. Pediatr. Pulmonol. 2001, 31, 67–75. [Google Scholar] [CrossRef]
- Zachariah, S.; Gharahbaghian, L.; Perera, P.; Joshi, N. Spontaneous pneumomediastinum on bedside ultrasound: Case report and review of the literature. West. J. Emerg. Med. 2015, 16, 321–324. [Google Scholar] [CrossRef]
- Scialanga, B.; Buonsenso, D.; Scateni, S.; Valentini, P.; Schingo, P.M.S.; Boccuzzi, E.; Mesturino, M.A.; Ferro, V.; Chiaretti, A.; Villani, A.; et al. Lung ultraound to detect pneumothorax in children evaluated for acute chest pain in the emergency department: An observational pilot study. Preprints 2021, 10, 2021010159. [Google Scholar]
- Wang, D.; Hu, B.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; Zhao, Y.; et al. Clinical charateristics of 138 hospitalized patients with novel 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 106–107. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Bellini, D.; Lichtner, M.; Vicini, S.; Rengo, M.; Ambrogi, C.; Carbone, L. Spontaneous pneumomediastinum as the only CT finding in an asymptomatic adolescent positive for COVID-19. BJR Case Rep. 2020, 6, 20200051. [Google Scholar] [CrossRef] [PubMed]
- Macia, I.; Moya, J.; Ramos, R.; Morera, R.; Escobar, I.; Saumench, J.; Perna, V.; Rivas, F. Spontaneous mediastinum–41 cases. Eur. J. Cardiothorac. Surg. 2007, 31, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.; Ali, S.Z.; Braud, R. Spontaneous pneumomediastinum: A comparative study and review of the literature. Ann. Thor. Surg. 2008, 86, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Leung, Y.Y.; Hui, J.Y.H.; Chan, V.L.; Leung, W.S.; Law, K.I.; Chan, C.S.; Chan, K.S. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur. Respir. J. 2004, 23, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Gatto, A.; Graglia, B.; Rivetti, S.; Ferretti, S.; Paradiso, F.V.; Chiaretti, A. Early spontaneous pneumothorax, pneumomediastinum and pneumorrhachis in an adolescent with SARS-CoV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4413–4417. [Google Scholar]
- DoLago, V.C.; Cezare, T.J.; Fortaleza, C.M.C.B.; Okoshi, M.P.; Baldi, B.G.; Tanni, S.E. Does COVID-19 increase the risk for spontaneous pneumomediastinum? Am. J. Med. Sci. 2020, 360, 735–737. [Google Scholar] [CrossRef]
- Jafari, J.; Jahani, Z.; Alikhani, R.; Alinaghi, S.A.S.; Hasannezhad, M.; Salahshour, F.; Asadollahi-Amin, A. Spontaneous Loculated Pneumomediastinum in a COVID-19-Infected Patient. Case Rep. Infect. Dis. 2022, 2022, 5943221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, F.; Terteliu Baitan, M.; Caliman Sturdza, O.A.; Gheorghita Puscaselu, R.; Filip, R. Spontaneous Pneumomediastinum in a 16-Year-Old Patient with SARS-CoV-2 Infection: A North-East Romanian Case. Children 2022, 9, 1641. https://doi.org/10.3390/children9111641
Filip F, Terteliu Baitan M, Caliman Sturdza OA, Gheorghita Puscaselu R, Filip R. Spontaneous Pneumomediastinum in a 16-Year-Old Patient with SARS-CoV-2 Infection: A North-East Romanian Case. Children. 2022; 9(11):1641. https://doi.org/10.3390/children9111641
Chicago/Turabian StyleFilip, Florin, Monica Terteliu Baitan, Olga Adriana Caliman Sturdza, Roxana Gheorghita Puscaselu, and Roxana Filip. 2022. "Spontaneous Pneumomediastinum in a 16-Year-Old Patient with SARS-CoV-2 Infection: A North-East Romanian Case" Children 9, no. 11: 1641. https://doi.org/10.3390/children9111641
APA StyleFilip, F., Terteliu Baitan, M., Caliman Sturdza, O. A., Gheorghita Puscaselu, R., & Filip, R. (2022). Spontaneous Pneumomediastinum in a 16-Year-Old Patient with SARS-CoV-2 Infection: A North-East Romanian Case. Children, 9(11), 1641. https://doi.org/10.3390/children9111641









