Changes in Nutritional State and Cardiovascular Parameters in Alimentary Obese Children after a Month-Long Stay in Children’s Treatment Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Anthropometric Parameters
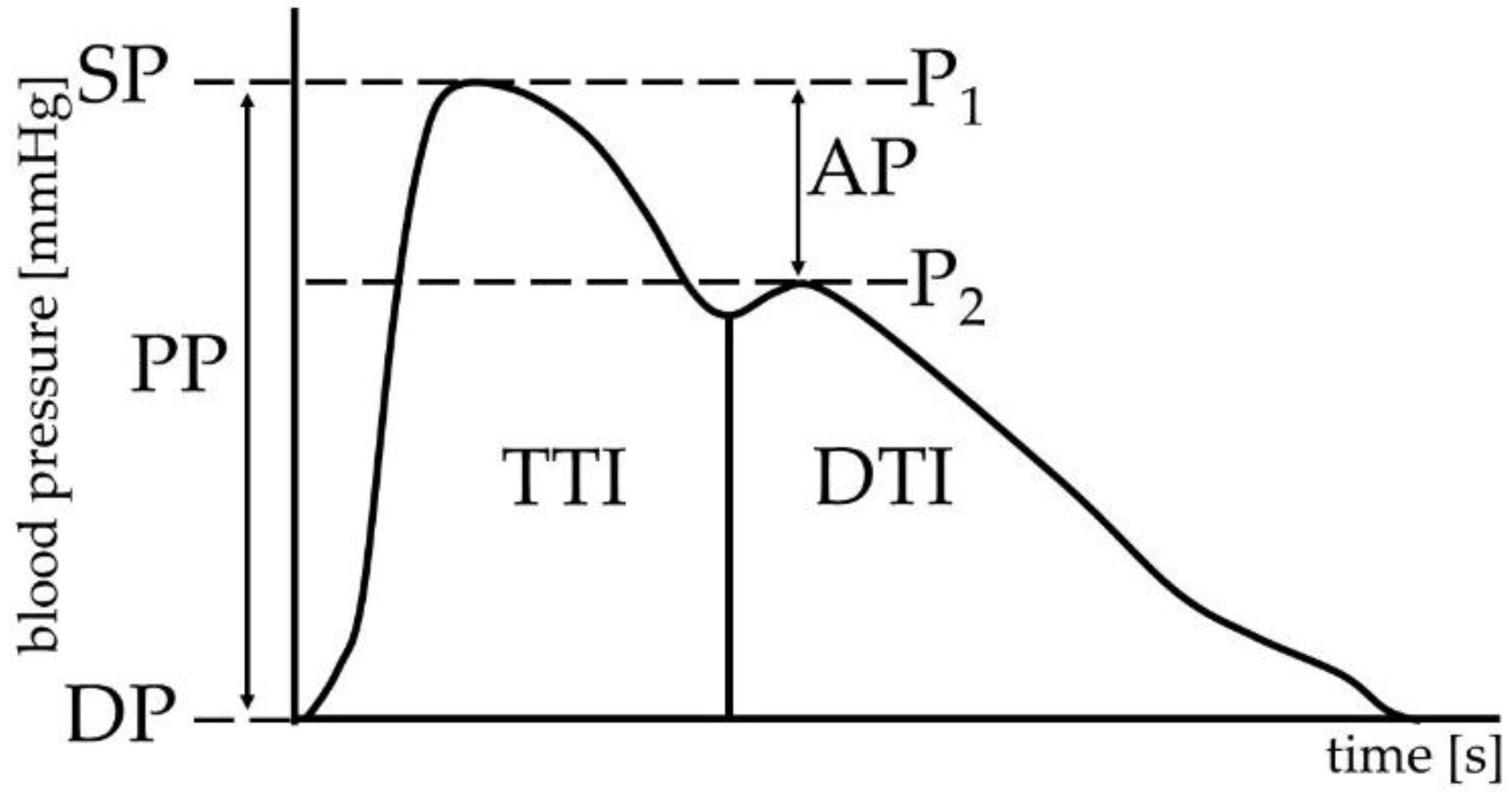
2.4. Cardiovascular Parameters
2.5. Statistical Analysis
3. Results
3.1. Anthropometry
3.1.1. Anthropometric Comparison of the Whole Cohort
3.1.2. Anthropometric Comparison of Sexes within the Age Groups
- Age group 12–14 years: BWTI (79.9 vs. 67.5 kg; p < 0.05), BMII (30.7 vs. 24.9 kg/m2; p < 0.05), BMIII (28.9 vs. 23.1 kg/m2; p < 0.05), WHRII (0.9 vs. 0.8; p < 0.01).
- Age group 15–17 years: FatI (30.3 vs. 45.7%; p < 0.01), FatII (28 vs. 43.8%; p < 0.01), WHRII (0.9 vs. 0.8; p < 0.05).
3.2. Cardiovascular Parameters
3.2.1. Comparison of Cardiovascular Parameters of the Whole Cohort
3.2.2. Comparison of Cardiovascular Parameters of Both Sexes within Every Age Group
3.2.3. Comparison of Cardiovascular Parameters between the Measurements within Every Age Group
4. Discussion
4.1. Anthropometry
4.2. Cardiovascular Parameters
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 16 July 2022).
- Pastucha, D.; Filipčíková, R.; Horáková, D.; Radová, L.; Marinov, Z.; Malinčíková, J.; Kocvrlich, M.; Horák, S.; Bezdičková, M.; Dobiáš, M. The Incidence of Metabolic Syndrome in Obese Czech Children: The Importance of Early Detection of Insulin Resistance Using Homeostatic Indexes HOMA-IR and QUICKI. Physiol. Res. 2013, 62, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Jain, V. Obesity in Children: Definition, Etiology and Approach. Indian J. Pediatr. 2018, 85, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch-Blüher, S.; Wiegand, S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018, 7, 254–259. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arter. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular Adipose Tissue Inflammation in Vascular Disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef]
- Dangardt, F.; Charakida, M.; Georgiopoulos, G.; Chiesa, S.T.; Rapala, A.; Wade, K.H.; Hughes, A.D.; Timpson, N.J.; Pateras, K.; Finer, N.; et al. Association between Fat Mass through Adolescence and Arterial Stiffness: A Population-Based Study from The Avon Longitudinal Study of Parents and Children. Lancet Child Adolesc. Health 2019, 3, 474–481. [Google Scholar] [CrossRef]
- Correia-Costa, A.; Correia-Costa, L.; Caldas Afonso, A.; Schaefer, F.; Guerra, A.; Moura, C.; Mota, C.; Barros, H.; Areias, J.C.; Azevedo, A. Determinants of Carotid-Femoral Pulse Wave Velocity in Prepubertal Children. Int. J. Cardiol. 2016, 218, 37–42. [Google Scholar] [CrossRef]
- Seznam Růstových Grafů Ke Stažení, SZÚ. Available online: http://www.szu.cz/publikace/data/seznam-rustovych-grafu-ke-stazeni (accessed on 16 July 2022).
- Marchesini Reggiani, G.; Dalle Grave, R.; Centis, E.; Marzocchi, R.; El Ghoch, M. Major Factors for Facilitating Change in Behavioral Strategies to Reduce Obesity. Psychol. Res. Behav. Manag. 2013, 6, 101–110. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Pietrabissa, G.; Manzoni, G.M.; Cattivelli, R.; Rossi, A.; Novelli, M.; Varallo, G.; Molinari, E. Cognitive Behavioral Therapy to Aid Weight Loss in Obese Patients: Current Perspectives. Psychol. Res. Behav. Manag. 2017, 10, 165–173. [Google Scholar] [CrossRef]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A Novel Blood Pressure-Independent Arterial Wall Stiffness Parameter; Cardio-Ankle Vascular Index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Svačinová, J.; Hrušková, J.; Jakubík, J.; Budinskaya, K.; Hidegová, S.; Fabšík, M.; Sieglová, H.; Kaščáková, Z.; Novák, J.; Nováková, Z. Variability of Peripheral Pulse Wave Velocity in Patients With Diabetes Mellitus Type 2 During Orthostatic Challenge. Physiol. Res. 2020, 69, S433–S441. [Google Scholar] [CrossRef] [PubMed]
- Butlin, M.; Qasem, A. Large Artery Stiffness Assessment Using SphygmoCor Technology. Pulse 2017, 4, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Stabouli, S.; Printza, N.; Zervas, C.; Dotis, J.; Chrysaidou, K.; Maliahova, O.; Antza, C.; Papachristou, F.; Kotsis, V. Comparison of the SphygmoCor XCEL Device with Applanation Tonometry for Pulse Wave Velocity and Central Blood Pressure Assessment in Youth. J. Hypertens. 2019, 37, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Svacinová, J.; Moudr, J.; Honzíková, N. Baroreflex sensitivity: Diagnostic importance, methods of determination and a model of baroreflex blood-pressure regulation. Ceskoslovenska Fysiol. 2013, 62, 10–18. [Google Scholar]
- Kelly, D.M.; Jones, T.H. Testosterone and Obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Grantham, J.P.; Henneberg, M. The Estrogen Hypothesis of Obesity. PLoS ONE 2014, 9, e99776. [Google Scholar] [CrossRef]
- Kaplowitz, P.B. Link Between Body Fat and the Timing of Puberty. Pediatrics 2008, 121, S208–S217. [Google Scholar] [CrossRef]
- Nicolaidis, S. Environment and Obesity. Metabolism 2019, 100, 153942. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, X.; Wu, H.; Chai, X.; Yang, X. Trends in Overweight and Obesity Among Children and Adolescents in China from 1991 to 2015: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4656. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef] [PubMed]
- Stages of Puberty: A Guide for Males and Females. Available online: https://www.healthline.com/health/parenting/stages-of-puberty (accessed on 17 July 2022).
- Hertiš, T.; Petek, T.; Marčun Varda, N. The Prevalence of Elevated Blood Pressure in a Sample of Slovene Children and Adolescents: A Pilot Study. Slov. J. Public Health 2018, 57, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.S.; Devlin, A.M. Childhood Obesity and Cardiovascular Dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac Remodeling in Obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Blackett, P.R.; Gardner, A.W.; Copeland, K.C. Vascular Health in Children and Adolescents: Effects of Obesity and Diabetes. Vasc. Health Risk Manag. 2009, 5, 973–990. [Google Scholar] [CrossRef]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased Rate of Arterial Stiffening with Obesity in Adolescents: A Five-Year Follow-up Study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.D.; Rapala, A.; Khan, T.; Williams, B.; Viner, R.M. Evidence for Contemporary Arterial Stiffening in Obese Children and Adolescents Using Pulse Wave Velocity: A Systematic Review and Meta-Analysis. Atherosclerosis 2015, 241, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Tryggestad, J.B.; Short, K.R. Arterial Compliance in Obese Children: Implications for Cardiovascular Health. Exerc. Sport Sci. Rev. 2014, 42, 175–182. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. The Role of Vagal Function in the Risk for Cardiovascular Disease and Mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef]
- Schäfer, A.; Vagedes, J. How Accurate Is Pulse Rate Variability as an Estimate of Heart Rate Variability? A Review on Studies Comparing Photoplethysmographic Technology with an Electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C. Childhood Obesity and Cardiac Remodeling: From Cardiac Structure to Myocardial Mechanics. J. Cardiovasc. Med. 2015, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A. Obesity Cardiomyopathy: Pathophysiology and Evolution of the Clinical Syndrome. Am. J. Med. Sci. 2001, 321, 225–236. [Google Scholar] [CrossRef]
- Peterson, L.R.; Waggoner, A.D.; Schechtman, K.B.; Meyer, T.; Gropler, R.J.; Barzilai, B.; Dávila-Román, V.G. Alterations in Left Ventricular Structure and Function in Young Healthy Obese Women: Assessment by Echocardiography and Tissue Doppler Imaging. J. Am. Coll. Cardiol. 2004, 43, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Pacileo, G.; Del Giudice, E.M.; Natale, F.; Limongelli, G.; Verrengia, M.; Rea, A.; Fratta, F.; Castaldi, B.; D’Andrea, A.; et al. Abnormal Myocardial Deformation Properties in Obese, Non-Hypertensive Children: An Ambulatory Blood Pressure Monitoring, Standard Echocardiographic, and Strain Rate Imaging Study. Eur. Heart J. 2006, 27, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Lorch, S.M.; Sharkey, A. Myocardial Velocity, Strain, and Strain Rate Abnormalities in Healthy Obese Children. J. Cardiometab. Syndr. 2007, 2, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Pulenthiran, A.; Nevius, C.D.; Mejia-Spiegeler, A.; Suever, J.D.; Wehner, G.J.; Kirchner, H.L.; Haggerty, C.M.; Fornwalt, B.K. Impaired Right Ventricular Contractile Function in Childhood Obesity and Its Association with Right and Left Ventricular Changes: A Cine DENSE Cardiac Magnetic Resonance Study. J. Cardiovasc. Magn. Reson. 2017, 19, 49. [Google Scholar] [CrossRef]
- Šudaková, M.; Budinskaya, K.; Nováková, Z. Evaluation of Diastolic Heart Function Using Echocardiography and Pulse Wave Analysis in Patients After Anthracycline Therapy. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021; Volume 48, pp. 1–4. [Google Scholar]
- Marčun-Varda, N.; Nikolic, S.; Močnik, M. Subendocardial Viability Ratio and Ejection Duration as Parameters of Early Cardiovascular Risk in Children. Clin. Nephrol. 2017, 88, 35–38. [Google Scholar] [CrossRef]
- Yang, H.; Huynh, Q.L.; Venn, A.J.; Dwyer, T.; Marwick, T.H. Associations of Childhood and Adult Obesity with Left Ventricular Structure and Function. Int. J. Obes. 2017, 41, 560–568. [Google Scholar] [CrossRef]
- Lalan, S.; Blowey, D. Comparison between Oscillometric and Intra-Arterial Blood Pressure Measurements in Ill Preterm and Full-Term Neonates. J. Am. Soc. Hypertens. 2014, 8, 36–44. [Google Scholar] [CrossRef]
- Lu, B.; Zhou, J.; Waring, M.E.; Parker, D.R.; Eaton, C.B. Abdominal Obesity and Peripheral Vascular Disease in Men and Women: A Comparison of Waist-to-Thigh Ratio and Waist Circumference as Measures of Abdominal Obesity. Atherosclerosis 2010, 208, 253–257. [Google Scholar] [CrossRef]
- Casey, S.; Lanting, S.; Oldmeadow, C.; Chuter, V. The Reliability of the Ankle Brachial Index: A Systematic Review. J. Foot Ankle Res. 2019, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.E.; Berg, R.L.; Economos, C.; Coleman, L.A. The Relationship between Childhood BMI and Adult Serum Cholesterol, LDL, and Ankle Brachial Index. Clin. Med. Res. 2014, 12, 33–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doraiswamy, V.A.; Giri, J.; Mohler, E. Premature Peripheral Arterial Disease–Difficult Diagnosis in Very Early Presentation. Int. J. Angiol. 2009, 18, 45–47. [Google Scholar] [CrossRef] [PubMed][Green Version]


| BWT (kg) | BMI (kg/m2) | SP (mmHg) | DP (mmHg) | ||
|---|---|---|---|---|---|
| Whole cohort | 76 (63–89) | 28.6 (25–31.6) | 126 (117–139) | 70 (64–75) | |
| 9–11 years | Males | 63 (60–67) | 27.9 (27.2–29) | 116 (115–123) | 65 (63–74) |
| Females | 55.7 (50–74.2) | 26.1 (24.4–30.3) | 115 (112–127) | 66 (64–75) | |
| 12–14 years | Males | 80 (72–91) | 30.7 (26–31) | 137 (130–145) | 70 (65–74) |
| Females | 68 (66–78) | 24.9 (24–30) | 124 (122–125) | 66 (66–74) | |
| 15–17 years | Males | 96.6 (77.8–105.5) | 30 (27.3–33) | 142 (133–146) | 72 (70–75) |
| Females | 98.4 (92–100.5) | 35.4 (33.4–38.9) | 136 (123–138) | 80 (73–80) | |
| Parameter | Measurement I. | Measurement II. | p Value |
|---|---|---|---|
| BWT (kg) | 75.5 (63.4–89.4) | 72.6 (61.8–86.9) | p < 0.01 |
| BMI (kg/m2) | 28.6 (25.4–31.6) | 26.9 (24.2–30.3) | p < 0.01 |
| Fat (%) | 36.9 (32.2–42.3) | 34.5 (28.9–39.2) | p < 0.01 |
| WC (cm) | 87.5 (82.5–95.5) | 88 (80–92.5) | p < 0.01 |
| HC (cm) | 101.5 (95.5–111.5) | 99.5 (95–109.5) | p < 0.01 |
| WHR | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | NS |
| Male | Female | p Value (M vs. F) | ||
|---|---|---|---|---|
| 9–11 years | Physiological range | 12–23% | 16–28% | |
| Measurement I. | 40.5 (36.4–43.5) | 37 (36.2–38.9) | NS | |
| Measurement II. | 38.5 (36.6–40.8) | 35.4 (32–37.6) | NS | |
| p value (I. vs. II.) | NS | p < 0.05 | ||
| 12–14 years | Physiological range | 11–22% | 16–29% | |
| Measurement I. | 35 (31.6–41.3) | 35.4 (31.5–38.7) | NS | |
| Measurement II. | 30.1 (27.4–35.8) | 30.9 (26.4–37.5) | NS | |
| p value (I. vs. II.) | p < 0.01 | NS | ||
| 15–17 years | Physiological range | 10–20% | 16–30% | |
| Measurement I. | 30.5 (23.7–36.1) | 45.7 (43.3–47.1) | p < 0.05 | |
| Measurement II. | 28 (24.5–34.2) | 43.8 (39.6–45.5) | p < 0.05 | |
| p value (I. vs. II.) | NS | NS | ||
| Parameter | Measurement I. | Measurement II. | p Value |
|---|---|---|---|
| SP (mmHg) | 108 (100–117) | 107 (100–112) | NS |
| DP (mmHg) | 64 (60–71.5) | 62 (58.5–69) | p < 0.05 |
| AP (mmHg) | 0 (−6–7) | 0 (−8–4) | NS |
| AI (%) | 97 (91.5–104) | 98 (94–106) | NS |
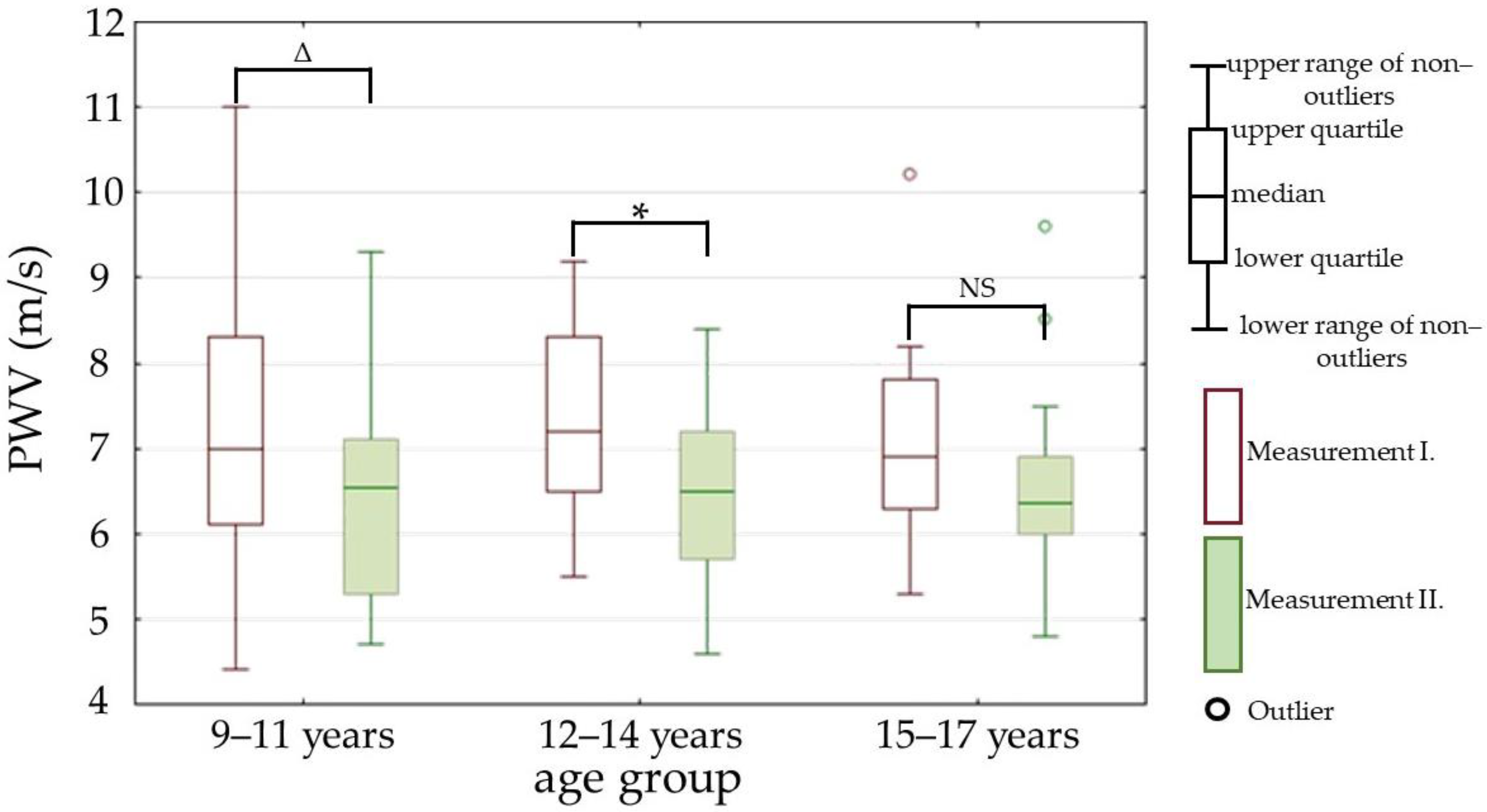
| PWV (m/s) | 7.1 (6.3–8.2) | 6.5 (5.7–7.2) | p < 0.01 |
| PWVV (m/s) | 0.6 (0.5–0.7) | 0.6 (0.4–0.8) | NS |
| CAVI | 4.3 (3.6–5) | 4.3 (3.8–4.9) | NS |
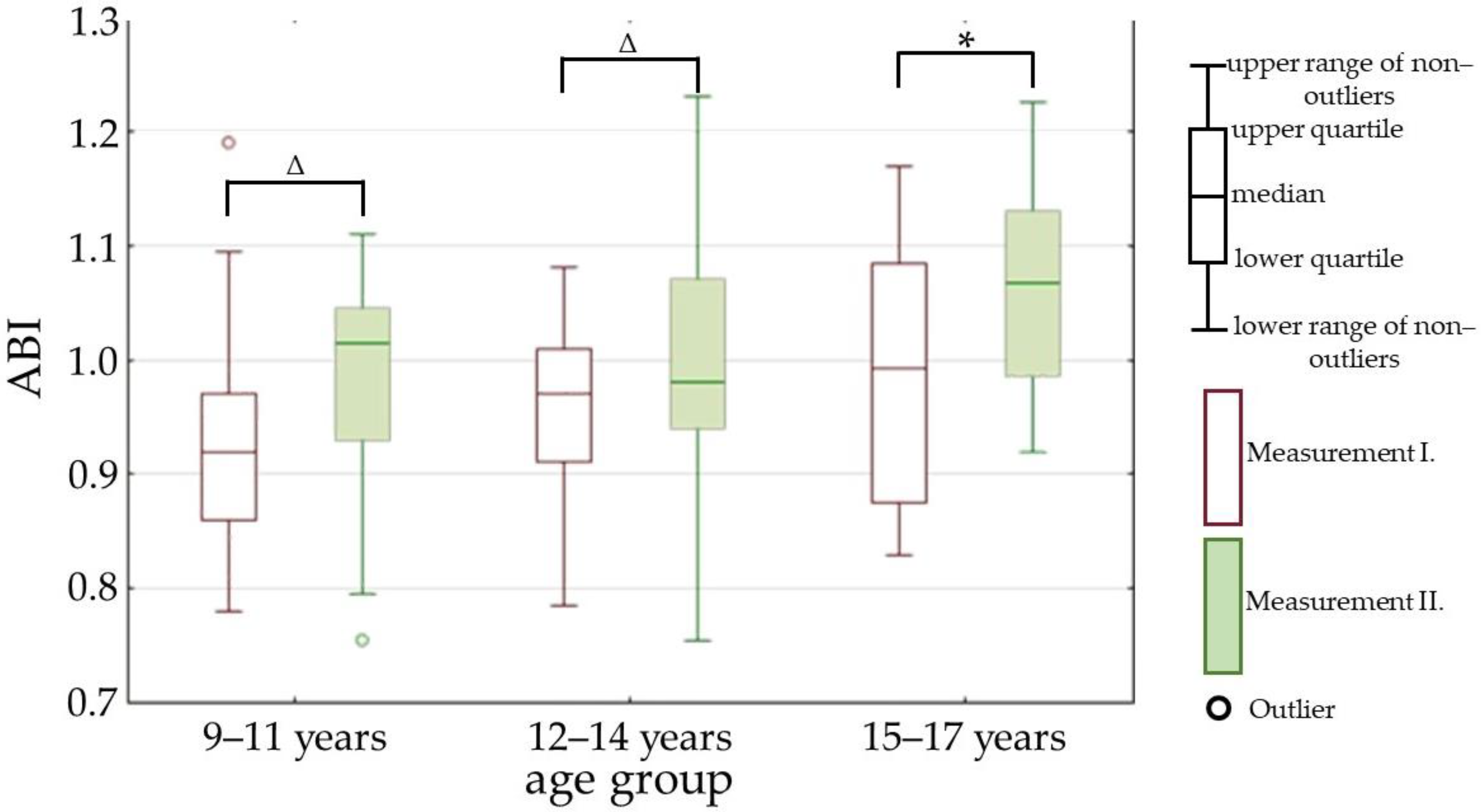
| ABI | 0.96 (0.88–1.01) | 1.02 (0.94–1.07) | p < 0.01 |
| TTI | 1910 (1770–2075) | 1710.5 (1499.5–1888) | p < 0.01 |
| DTI | 2758.5 (2618.5–3064) | 2934.5 (2756–3127) | NS |
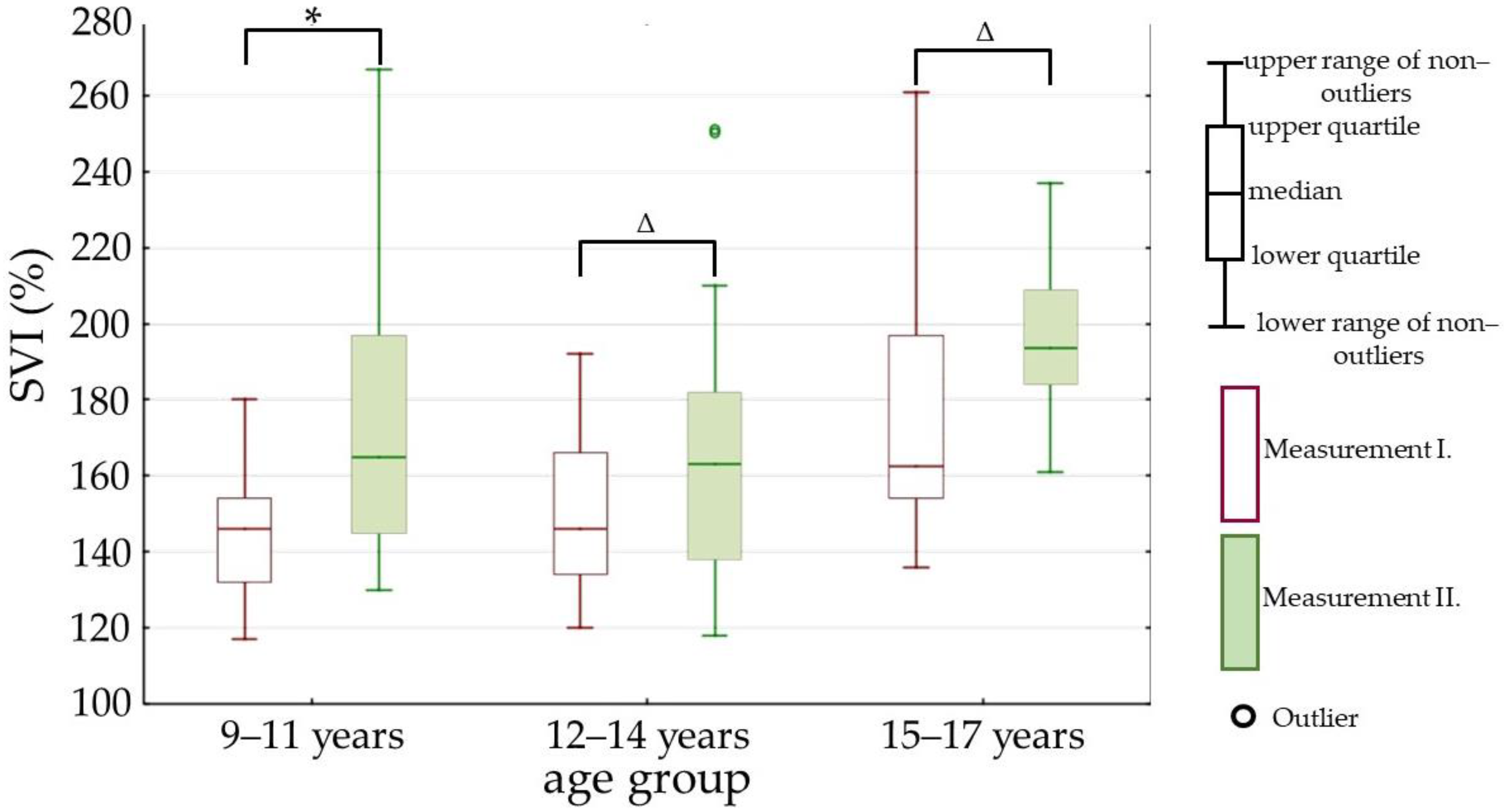
| SVI (%) | 151 (136–166.5) | 173.5 (146.5–198) | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budinskaya, K.; Pírek, O.; Rafčíková, N.; Nádeníčková, O.; Bednaříková, K.; Hrstková, H.; Dobšák, P.; Nováková, Z. Changes in Nutritional State and Cardiovascular Parameters in Alimentary Obese Children after a Month-Long Stay in Children’s Treatment Center. Children 2022, 9, 1610. https://doi.org/10.3390/children9111610
Budinskaya K, Pírek O, Rafčíková N, Nádeníčková O, Bednaříková K, Hrstková H, Dobšák P, Nováková Z. Changes in Nutritional State and Cardiovascular Parameters in Alimentary Obese Children after a Month-Long Stay in Children’s Treatment Center. Children. 2022; 9(11):1610. https://doi.org/10.3390/children9111610
Chicago/Turabian StyleBudinskaya, Ksenia, Ondřej Pírek, Natálie Rafčíková, Olga Nádeníčková, Kateřina Bednaříková, Hana Hrstková, Petr Dobšák, and Zuzana Nováková. 2022. "Changes in Nutritional State and Cardiovascular Parameters in Alimentary Obese Children after a Month-Long Stay in Children’s Treatment Center" Children 9, no. 11: 1610. https://doi.org/10.3390/children9111610
APA StyleBudinskaya, K., Pírek, O., Rafčíková, N., Nádeníčková, O., Bednaříková, K., Hrstková, H., Dobšák, P., & Nováková, Z. (2022). Changes in Nutritional State and Cardiovascular Parameters in Alimentary Obese Children after a Month-Long Stay in Children’s Treatment Center. Children, 9(11), 1610. https://doi.org/10.3390/children9111610





