Current Management of Generalized Convulsive Status Epilepticus in Children
Abstract
1. Introduction
1.1. Epidemiology
1.2. Pathophysiology and Etiopathogenesis
1.3. Definition
- ►
- t1—The time at which spontaneous termination of epileptic activity is unlikely and adequate therapy should be initiated at this point at the latest;
- ►
- t2—The time beyond which continued epileptic activity can cause long-term pathological changes in the brain (alteration of neuronal network function, neuronal damage, neuronal death).
1.4. Classification
- ►
- Focal SE without impaired consciousness—continuous or recurrent focal motor or sensory seizure activity without alteration of consciousness;
- ►
- Focal SE with impaired consciousness—continuous or recurrent focal motor (automatisms) or sensory seizure activity with altered consciousness;
- ►
- Generalized convulsive SE—tonic-clonic, tonic, or clonic convulsions always associated with unconsciousness;
- ►
- Absence of SE—generalized seizure activity characterized by alteration of consciousness, but not always unconsciousness;
- ►
- Myoclonic SE—continuous myoclonia can occur in diseases of a wide spectrum of severity, from benign epileptic syndromes (juvenile myoclonic epilepsy) to conditions with poor prognosis (postanoxic encephalopathy).
1.5. Notes on Semiological Classification:
- ►
- Generalized convulsive seizures are divided into primarily generalized and focal seizures transitioning into a bilateral tonic-clonic seizure (focal bilateral tonic-clonic seizure). These arise from the generalization of the seizure activity of the epileptogenic focus. Clinically, the two types usually cannot be distinguished (generalization usually occurs so quickly that no focal symptoms manifest themselves);
- ►
- Absence SE and focal SE (with or without impaired consciousness) without motor symptomatology are denoted by the term non-convulsive SE (also subclinical SE). Non-convulsive SE can only be diagnosed electroencephalographically;
- ►
- Non-convulsive SE may also be followed by untreated or inadequately treated generalized convulsive SE. In this case, it is called subtle SE. Patients tend to be comatose, possibly with inconspicuous motor symptoms, such as minor generalized myoclonia or twitching of the eyeballs. Subtle SE is the non-convulsive epileptic state with the worst prognosis. Other authors have excluded subtle SE from the group of non-convulsive SEs.
1.6. Etiopathogenesis
- ►
- Hypoxic-ischemic encephalopathy;
- ►
- Intracerebral hemorrhage;
- ►
- Neuroinfection (pre-, peri-, post-natal);
- ►
- Cortical dysplasia and CNS malformations (disorders of migration, segmentation, myelination, synaptogenesis, etc.);
- ►
- Metabolic causes (hypocalcemia, hypoglycemia, hypomagnesemia, hyponatremia);
- ►
- Febrile status epilepticus (neuroinfection must be excluded);
- ►
- Some genetic syndromes, such as Dravet syndrome and Angelman syndrome, can be manifested by recurrent attacks of SE;
- ►
- Autoimmune encephalitis: Rasmussen’s encephalitis, new onset refractory status epilepticus (NORSE), febrile infection-related epileptic syndrome (FIRES), NMDA encephalitis;
- ►
- Rarely, metabolic epileptic encephalopathies (1–2%).
1.7. Diagnosis of the Etiology
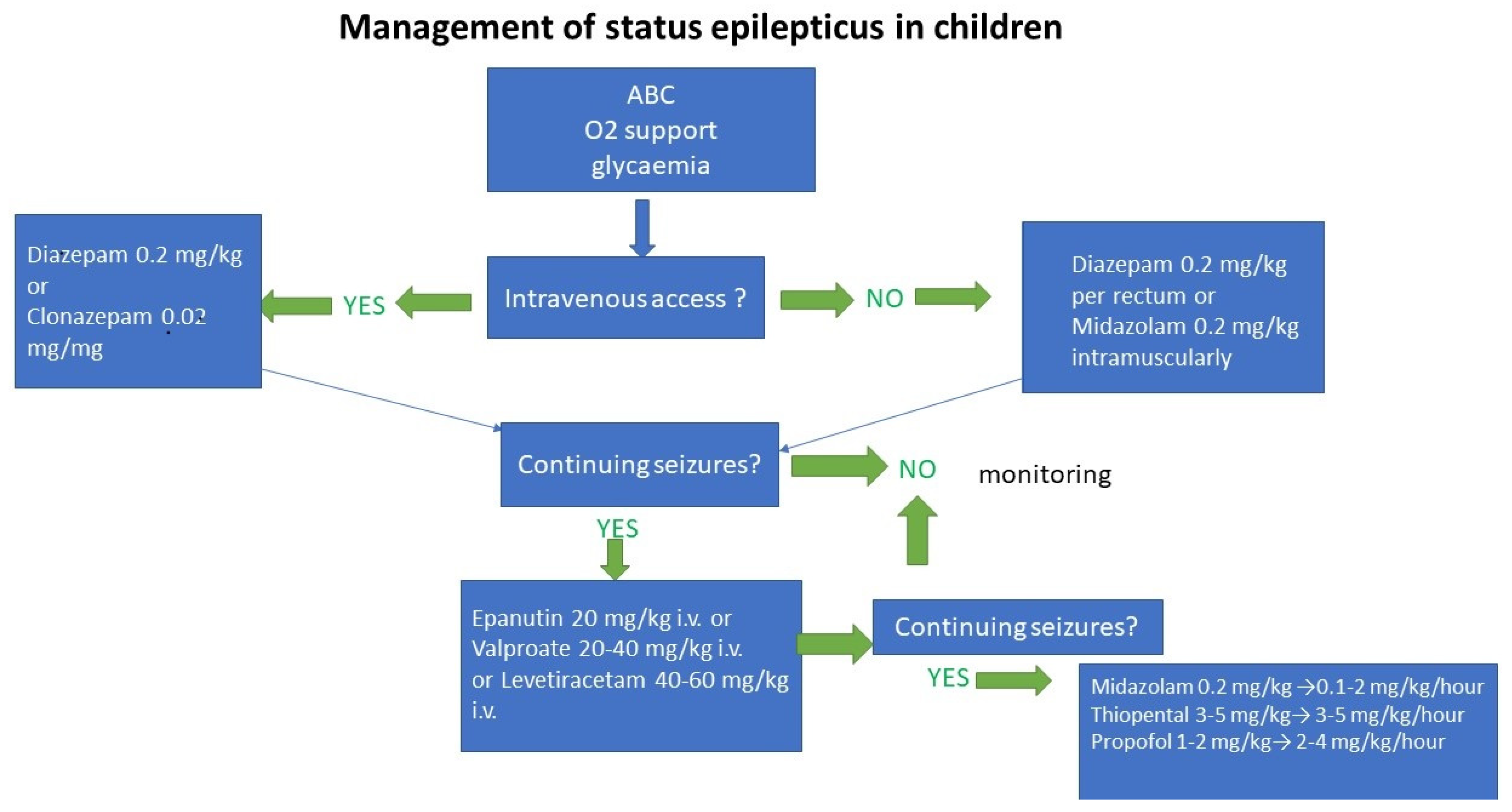
2. Management of the Treatment of Generalized Convulsive Status Epilepticus (GCSE)
2.1. Treatment in the Individual Stages of GCSE
2.2. Stages of GCSE
- ►
- Stage 1 (early GCSE; 5–20 min): initiation of general therapy, monitoring and diagnosis. Boluses of benzodiazepine. The time from 0–5 min is referred to as impending GCSE.
- ►
- Stage 2 (developed GCSE; 20–40 min): continued GCSE after administration of two benzodiazepines boluses, initiation of an infusion of a non-benzodiazepine antiepileptic drug.
- ►
- Stage 3 (refractory GCSE; >40 min): continued GCSE even after administration of adequate doses of benzodiazepine and non-benzodiazepine antiseizure medication (ASM), indication either for treatment with other non-benzodiazepine ASM or directly for intravenous general anesthesia.
- ►
- Stage 4 (super-refractory GCSE; >24 h): GCSE continues with general anesthesia, including situations in which there is recurrence of seizure after discontinuation or reduction of anesthesia.
3. Algorithms of GCSE Treatment in Individual Stages of GCSE
3.1. Impending GCSE (0–5 Min)
- ►
- Head elevation 30°;
- ►
- Oxygen therapy with O2 mask with reservoir;
- ►
- SpO2 and ECG monitoring (one lead), non-invasive blood pressure measurement;
- ►
- Ensuring venous entry;
- ►
- Collection of capillary blood for glycaemia; in cases of hypoglycemia, application of glucose bolus intravenously (in neonates 10% glucose, in larger children up to 40% glucose);
- ►
- Venous blood sampling for blood counts, biochemistry, antiepileptic levels (see above);
- ►
- Temperature control in case of fever or hyperthermia;
- ►
- Monitoring of vital signs continues throughout GCSE therapy;
- ►
- At any time (not only in the first five minutes) during GCSE therapy, impaired oxygenation (desaturation, cyanosis) and/or ventilation (hypoventilation, apnea) should lead to the introduction of general anesthesia, intubation, and ventilation
3.2. Early GCSE (5–20 Min)
3.3. Developed GCSE (20–40 Min)
- ►
- According to EpiStop recommendations, phenytoin is administered in children under 12 years of age at a saturation dose of 20–30 mg per kg intravenously and the rate of administration is recommended to be slower than in adults (25 mg/min) [7];
- ►
- Risk of hypotension and/or bradycardia (use caution in patients with heart disease—in this case, consider another second-choice medicine);
- ►
- Dilute to saline (not up to 5% glucose—this would precipitate phenytoin).
- ►
- Risk of seizure aggravation: Dravet syndrome, idiopathic generalized epilepsy (e.g., juvenile myoclonic epilepsy)—> use valproic acid or levetiracetam [9];
- ►
- Phenytoin may not be effective for convulsions during intoxication (convulsions caused by cocaine, local anesthetics, and theophylline may even worsen);
- ►
- In the U.S., fosphenytoin is used instead of phenytoin.
4. Refractory GCSE (>40 Min)
5. Propofol Infusion Syndrome
- ►
- Rhabdomyolysis with myoglobinuria, which secondarily leads to acute renal failure with hyperkalemia;
- ►
- Hepatomegaly or hepatic steatosis with elevation of liver enzymes;
- ►
- Hyperlipidemia or hypertriglyceridemia;
- ►
- Severe metabolic or lactate acidosis.
6. Super-Refractory GCSE (Lasting More Than 24 h)
- ►
- ►
- ►
- ►
- Pulse doses of methylprednisolone + IVIG +—plasmapheresis, if the autoimmune etiology of GCSE cannot be excluded [11,15]; e.g., if NMDA-receptor encephalitis is suspected; when this encephalitis is detected, the use of cyclophosphamide or rituximab may be considered—in case of failure of the immunotherapy mentioned above [15];
- ►
- Intravenous local anesthetic lidocaine (2 mg/kg followed by 2 mg/kg/h) [11];
- ►
- Inhaled anesthetic drugs—isoflurane or desflurane (not sevoflurane) [11]; although an effective solution when achieving adequate levels of inhalation anesthetic, there is a tendency to recurrence of GCSE during withdrawal;
- ►
- ►
- Rational cooling to a target central temperature of 34–35 °C (at temperatures <34 °C there is a risk of serious side effects);
- ►
- ►
- Epileptosurgical resection of a clearly proven epileptogenic focus in an operable area of the brain [11];
- ►
- Implantation of vagal nerve stimulation– in cases where epileptogenic focus cannot be clearly demonstrated [11];
- ►
- Various forms of stimulation therapy (transcranial magnetic stimulation, deep brain stimulus, electroconvulsive therapy) and liquor drainage [11] must be considered experimental;
- ►
7. Conclusions
8. Key Points
- (1)
- Generalized convulsive status epilepticus is emergency condition in children with high morbidity and mortality and potentially irreversible brain damage;
- (2)
- Early initiation and adequate treatment is crucial for good patient outcome;
- (3)
- Management of generalized convulsive status epilepticus depends on the stages of GCSE: early GCSE (5 to 20 min); developed GCSE (lasting 20 to 40 min); refractory GCSE (lasting more than 40 min); super-refractory GCSE (lasting more than 24 h);
- (4)
- Treatment must be initiated in the impending GCSE phase, within five minutes of the onset of a generalized convulsive seizure.
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| GCSE | generalized convulsive status epilepticus |
| SE | status epilepticus |
| GABA | gamma-aminobutyric acid |
| NMDA | N-methyl-D aspartate |
| NORSE | new onset refractory status epilepticus |
| FIRES | febrile infection-related epilepsy syndrome |
| PRIS | propofol infusion syndrome |
| EEG | electroencephalography |
| IVIG | intravenous immunoglobulins |
References
- Raspall-Chaure, M.; Chin, R.F.; Neville, B.G.; Bedford, H.; Scott, R.C. The epidemiology of convulsive status epilepticus in children: A critical review. Epilepsia 2007, 48, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Hanhan, U.A.; Fiallos, M.R.; Orlowski, J.P. Status epilepticus. Pediatr. Clin. N. Am. 2001, 48, 683–694. [Google Scholar] [CrossRef]
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Epistop. Available online: www.epistop.cz (accessed on 15 December 2018).
- Lansberg, M.G.; O’Brien, M.W.; Norbash, A.M.; Moseley, M.E.; Morrell, M.; Albers, G.W. MRI abnormalities associated with partial status epilepticus. Neurology 1999, 52, 1021. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Propofol Infuse Syndrome. Available online: https://lifeinthefastlane.com/ccc/propofol-infusion-syndrome/ (accessed on 30 March 2020).
- Management of Convulsive Status Epilepticus. Available online: https://www.uptodate.com/contents/management-of-convulsive-status-epilepticus-in-children (accessed on 23 November 2021).
- Juvenile Myoclonic Epilepsy. Available online: https://www.uptodate.com/contents/juvenile-myoclonic-epilepsy (accessed on 18 April 2022).
- Poddar, K.; Sharma, R.; Ng, Y.T. Intravenous Lacosamide in Pediatric Status Epilepticus: An Open-Label Efficacy and Safety Study. Pediatr. Neurol. 2016, 61, 83–86. [Google Scholar] [CrossRef]
- Shorvon, S.; Ferlisi, M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain 2011, 134, 2082–2818. [Google Scholar] [CrossRef]
- Loh, N.-H.W.; Nair, P. Propofol infusion syndrome. Contin. Educ. Anaesth. Criti. Care Pain 2013, 13, 200–202. [Google Scholar] [CrossRef]
- Status Epilepticus. Available online: https://emedicine.medscape.com/article/1164462-overview#a2 (accessed on 13 February 2018).
- Aroor, S.; Shravan, K.; Mundkur, S.; Jayakrishnan, C.; Rao, S. Super-refractory status epilepticus: A therapeutic challenge in paediatrics. J. Clin. Diagn. Res. 2017, 11, SR01–SR04. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Carpio, A.; Forsgren, L.; Hesdorffer, D.C.; Malmgren, K.; Sander, J.W.; Tomson, T.; Hauser, W.A. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010, 51, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Broomall, E.; Natale, J.E.; Grimason, M.; Goldstein, J.; Smith, C.M.; Chang, C.; Kanes, S.; Rogawski, M.; Wainwright, M. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann. Neurol. 2014, 76, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Huh, L.; Korn, P.; Farrell, K. Guideline for the management of convulsive status epilepticus in infants and children. BCMJ 2011, 53, 279–285. [Google Scholar]
- Kenney-Jung, D.L.; Vezzani, A.; Kahoud, R.J.; LaFrance-Corey, R.G.; Ho, M.L.; Muskardin, T.W.; Wirrell, E.C.; Howe, C.L.; Payne, E.T. Febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol. 2016, 80, 939–945. [Google Scholar] [CrossRef] [PubMed]

| Population of Patients | Examinations |
|---|---|
| In all patients | Blood count and biochemical examination CT or MRI of the brain * EEG ** Toxicology screening in indicated cases |
| In patients with epilepsy | Levels of antiepileptic drugs according to pharmacological history |
| In febrile patients |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulická, Š. Current Management of Generalized Convulsive Status Epilepticus in Children. Children 2022, 9, 1586. https://doi.org/10.3390/children9101586
Aulická Š. Current Management of Generalized Convulsive Status Epilepticus in Children. Children. 2022; 9(10):1586. https://doi.org/10.3390/children9101586
Chicago/Turabian StyleAulická, Štefania. 2022. "Current Management of Generalized Convulsive Status Epilepticus in Children" Children 9, no. 10: 1586. https://doi.org/10.3390/children9101586
APA StyleAulická, Š. (2022). Current Management of Generalized Convulsive Status Epilepticus in Children. Children, 9(10), 1586. https://doi.org/10.3390/children9101586





