Neonatal Hyperglycemia and Neurodevelopmental Outcomes in Preterm Infants: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Hyperglycemic group: infants with at least 1 episode of hyperglycemia defined as blood glucose concentration ≥8 mmol/L treated or not with insulin;
- Control group: infants without any episodes of hyperglycemia.
2.2. Research Strategy
2.3. Data Extraction
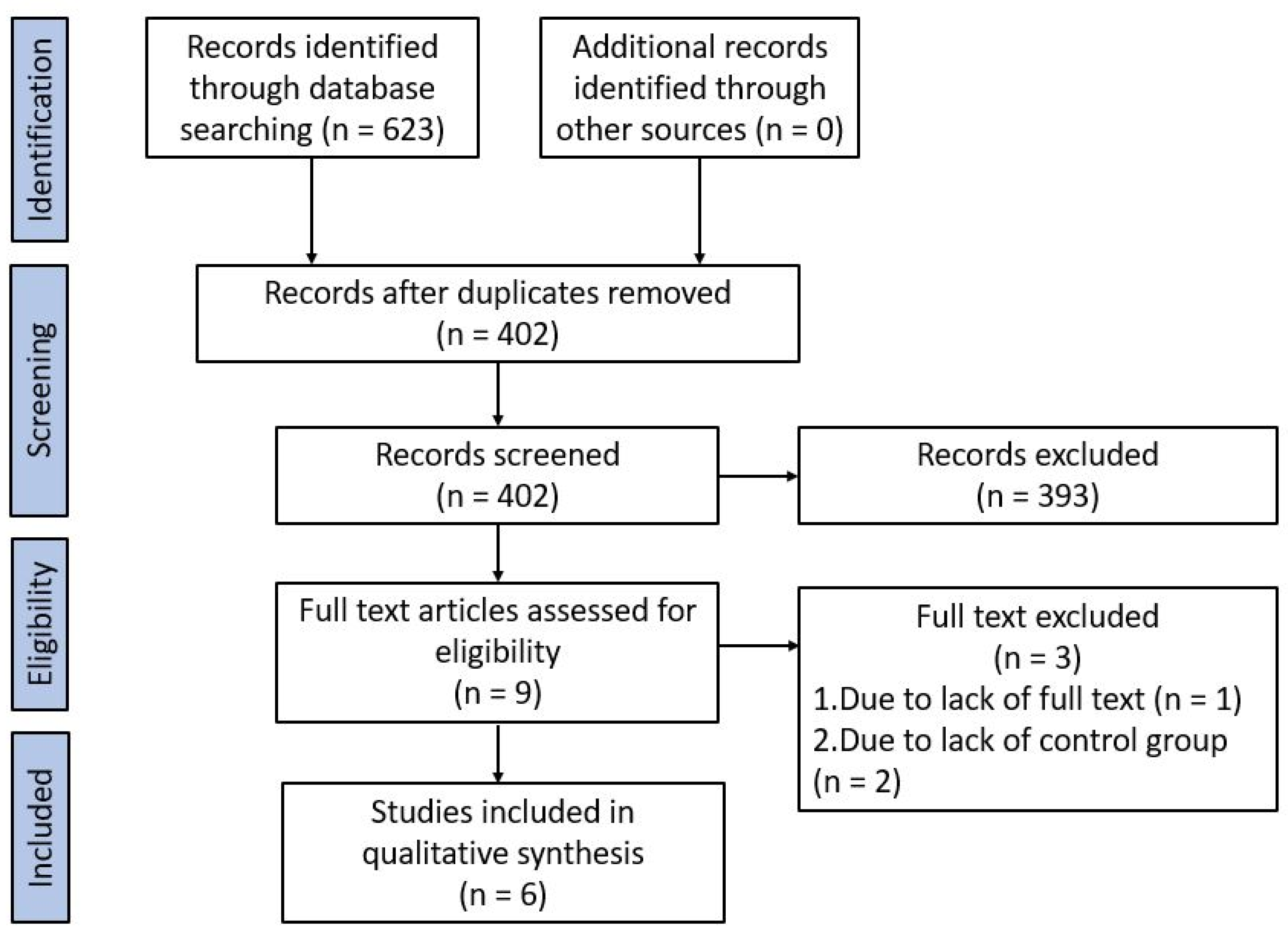
3. Results
3.1. Research Strategy
3.2. Characteristics of Selected Studies
3.3. Results of Individual Studies
3.3.1. Early Childhood
3.3.2. Later Childhood
3.3.3. Quality of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lawn, J.E.; Cousens, S.N.; Darmstadt, G.L.; Bhutta, Z.A.; Martines, J.; Paul, V.; Knippenberg, R.; Fogstad, H.; Lancet Neonatal Survival Series Steering Team. 1 year after The Lancet Neonatal Survival Series—Was the call for action heard? Lancet 2006, 367, 1541–1547. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Olsen, J.E.; Lee, K.J.; Spittle, A.J.; Opie, G.F.; Clark, M.; Boland, R.A.; Roberts, G.; Josev, E.K.; Davis, N.; et al. Victorian Infant Collaborative Study Group. Temporal Trends in Neurodevelopmental Outcomes to 2 Years After Extremely Preterm Birth. JAMA Pediatr. 2021, 175, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C.; Bodeau-Livinec, F.; Morgan, A.S.; Goffinet, F.; Marret, S.; et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ 2017, 358, j3448. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.P.; Smith, E.O.; Sunehag, A.L. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 2006, 118, 1811–1818. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Mohamed, S.; Murray, J.C.; Dagle, J.M.; Colaizy, T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC Pediatr. 2013, 13, 78. [Google Scholar] [CrossRef]
- Beardsall, K.; Vanhaesebrouck, S.; Ogilvy-Stuart, A.L.; Vanhole, C.; Palmer, C.R.; Ong, K.; van Weissenbruch, M.; Midgley, P.; Thompson, M.; Thio, M.; et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: Cohort analyses of the NIRTURE study. J. Pediatr. 2010, 157, 715–719.e1-3. [Google Scholar] [CrossRef]
- King, R.A.; Smith, R.M.; Dahlenburg, G.W. Long term postnatal development of insulin secretion in early premature infants. Early Hum. Dev. 1986, 13, 285–294. [Google Scholar] [CrossRef]
- Richardson, C.C.; Hussain, K.; Jones, P.M.; Persaud, S.; Lobner, K.; Boehm, A.; Clark, A.; Christie, M.R. Low levels of glucose transporters and K+ATP channels in human pancreatic beta cells early in development. Diabetologia 2007, 50, 1000–1005. [Google Scholar] [CrossRef]
- Lucas, A.; Bloom, S.R.; Aynsley-Green, A. Metabolic and endocrine events at the time of the first feed of human milk in preterm and term infants. Arch. Dis. Child. 1978, 53, 731–736. [Google Scholar] [CrossRef]
- Beardsall, K. Hyperglycaemia in the Newborn Infant. Physiology Verses Pathology. Front. Pediatr. 2021, 9, 641306. [Google Scholar] [CrossRef] [PubMed]
- Lilien, L.D.; Rosenfield, R.L.; Baccaro, M.M.; Pildes, R.S. Hyperglycemia in stressed small premature neonates. J. Pediatr. 1979, 94, 454–459. [Google Scholar] [CrossRef]
- Rath, C.P.; Shivamallappa, M.; Muthusamy, S.; Rao, S.C.; Patole, S. Outcomes of very preterm infants with neonatal hyperglycaemia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal 2021, 107, F1–F12. [Google Scholar] [CrossRef] [PubMed]
- Ertl, T.; Gyarmati, J.; Gaa’l, V.; Szabò, I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol. Neonate 2006, 89, 56–59. [Google Scholar] [CrossRef]
- Blanco, C.L.; Baillargeon, J.G.; Morrison, R.L.; Gong, A.K. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J. Perinatol. 2006, 26, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.S.; Morris, B.H.; Lally, K.P.; Stewart, C.D.; Huseby, V.; Kennedy, K.A. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J. Perinatol. 2006, 26, 730–736. [Google Scholar] [CrossRef]
- Van der Lugt, N.M.; Smits-Wintjens, V.; Van Zwieten, P.; Walther, F.J. Short and long term outcome of neonatal hyperglycemia in very preterm infants: A retrospective follow-up study. BMC Pediatr. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Long, J.D.; Gray, H.; Durrwachter-Erno, K.; Demerath, E.W.; Rao, R. Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infants. J. Perinatol. 2013, 33, 882–886. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Johnson, S.; Moore, T.; Marlow, N. Using the Bayley-III to assess neurodevelopmental delay: Which cut-off should be used? Pediatr. Res. 2014, 75, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Burnett, A. Assessing developmental delay in early childhood—Concerns with the Bayley-III scales. Clin. Neuropsychol. 2017, 31, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Naseh, N.; Gonzalez, K.T.; Vaz, T.; Ferreira, H.; Kaul, Y.F.; Johansson, M.; Agren, J.; Canto Moreira, N.; Hellstrom-Westas, L. Early hyperglycemia and brain MRI findings in very Preterm infants. In Foundation Acta Pædiatrica; International Perinatal Collegium: Edinburgh, UK, 2017. [Google Scholar] [CrossRef]
- Tottman, A.C.; Alsweiler, J.M.; Bloomfield, F.H.; Gamble, G.; Jiang, Y.; Leung, M.; Poppe, T.; Thompson, B.; Wouldes, T.A.; PIANO Study Group; et al. Long-Term Outcomes of Hyperglycemic Preterm Infants Randomized to Tight Glycemic Control. J. Pediatr. 2018, 193, 68–75.e1. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.; Abdel-Latif, M.E.; Kent, A.L. Insulin infusion for hyperglycaemia in very preterm infants appears safe with no effect on morbidity, mortality and long-term neurodevelopmental outcome. J. Matern. Fetal Neonatal Med. 2012, 25, 2415–2418. [Google Scholar] [CrossRef]
- Tottman, A.C.; Alsweiler, J.M.; Bloomfield, F.H.; Pan, M.; Harding, J.E. Relationship between Measures of Neonatal Glycemia, Neonatal Illness, and 2-Year Outcomes in Very Preterm Infants. J. Pediatr. 2017, 188, 115–121. [Google Scholar] [CrossRef]
- Villamizar, J.D.G.; Haapala, J.L.; Scheurer, J.M.; Rao, R.; Ramel, S.E. Relationships between Early Nutrition, Illness, and Later Outcomes among Infants Born Preterm with Hyperglycemia. J. Pediatr. 2020, 223, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zamir, I.; Stoltz Sjöström, E.; Ahlsson, F.; Hansen-Pupp, I.; Serenius, F.; Domellöf, M. Neonatal hyperglycaemia is associated with worse neurodevelopmental outcomes in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal 2021, 106, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef]
- Mesotten, D.; Joosten, K.; van Kempen, A.; Verbruggen, S.; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Carbohydrates. Clin. Nutr. 2018, 37, 2337–2343. [Google Scholar] [CrossRef]
- Burakevych, M.; McKinlay, C.J.D.; Harris, D.L.; Alsweiler, J.M.; Harding, J.E. Factors influencing glycaemic stability after neonatal hypoglycaemia and relationship to neurodevelopmental outcome. Sci. Rep. 2019, 9, 8132. [Google Scholar] [CrossRef]
- Adamkin, D.H.; Committee on Fetus and Newborn. Clinical Report-Postnatal Glucose Homeostasis in Late-Preterm and Term Infants. Pediatrics 2011, 127, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab. J. 2016, 40, 365–366. [Google Scholar] [CrossRef]
- Ramel, S.; Raghavendra, R. Hyperglycemia in Extremely Preterm Infants. NeoReviews 2020, 21, e89. [Google Scholar] [CrossRef]
- Paulsen, M.E.; Brown, S.J.; Satrom, K.M.; Scheurer, J.M.; Ramel, S.E.; Rao, R.B. Long-Term Outcomes after Early Neonatal Hyperglycemia in VLBW Infants: A Systematic Review. Neonatology 2021, 118, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Harding, J.; Brown, J.; McKinlay, C. Neonatal Glycaemia and Neurodevelopmental Outcomes: A Systematic Review and Meta-Analysis. Neonatology 2019, 115, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Alsweiler, J.M.; Harding, J.E.; Bloomfield, F.H. Tight glycemic control with insulin in hyperglycemic preterm babies: A randomized controlled trial. Pediatrics 2012, 129, 639–647. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Study Type | Place, Time | Population | Sample Size (Incidence of Hyperglycemia) | Definition of Hyperglycemia | Experimental Group/Control Group | Age at Follow-Up | Sample Size at Follow Up (Experimental Group) |
|---|---|---|---|---|---|---|---|---|
| Van der Lugt, 2010 [19] | Retrospective | Netherlands, 01.2002–12.2006 | ≤32 GA | 859 (8%) | at least 2 ≥ 10 mmol/L during a 12 h period. | Hyperglycemic/no hyperglycemic | 2 y ± 3 m CA | 96 (34%) |
| Heald, 2012 [26] | Retrospective | Australia, 01.2006–12.2008 | <29 GA | 97 (18%) | ≥10 mmol/L and significant glycosuria | insulin/no insulin | 1 y CA | 73 (12%) |
| Tottman, 2017 [27] | Retrospective | New Zeland, 07.2005–10.2008 | <30 GA/BW <1500 g | 360 (20%) | ≥8.6 mmol/L on ≥2 measures >1 h apart or any >10 mmol/L during the first 7 DOL | Hyperglycemic/normoglycemic | 2 y CA | 280 (20%) |
| Villamizar, 2020 [28] | Retrospective | USA, 02.2012–06.2016 | BW <1500 g | 97 (48%) | 1 or more >8.2 mmol/L | Hyperglycemic/normoglycemic | 1 y CA | 66 (49%) |
| Zamir, 2021 [29] | Retrospective | Sweden, 04.2004–03.2007 | <27 GA | 533 (69%) | Four categories: >8–10–12–14 mmol/L at least 1 or 2–3 consecutive days during the first 28 DOL | Hyperglycemic/no hyperglycemic | 6.5 y ± 3 m | 436 (87%) |
| Boscarino, 2021 [30] | Prospective | Italy, 01.2015–12.2019 | <32 GA/BW <1500 g | 280 (29%) | 2 consecutive >10 mmol/L at least 3 h apart | Hyperglicemic/no hyperglicemic | 2 y CA | 108 (30%) |
| Neurodevelopmental Assessment Tool | Risk of Neurological Impairment in the Hyperglycemic Population | |||||
|---|---|---|---|---|---|---|
| Van der Lugt [19] | Heald [26] | Tottman [27] | Villamizar [28] | Zamir [29] | Boscarino [30] | |
| BSID | ↔ | ↑ * | ↑ ** | ↑ *** | ||
| GMDS | ↔ | |||||
| WISC | ↑ | |||||
| MABC | ↑ | |||||
| CP | ↔ | ↑ * | ↔ | |||
| B or D | ↔ | ↑ * | ↔ | |||
| Other | ↑ | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiducci, S.; Meggiolaro, L.; Righetto, A.; Piccoli, M.; Baraldi, E.; Galderisi, A. Neonatal Hyperglycemia and Neurodevelopmental Outcomes in Preterm Infants: A Review. Children 2022, 9, 1541. https://doi.org/10.3390/children9101541
Guiducci S, Meggiolaro L, Righetto A, Piccoli M, Baraldi E, Galderisi A. Neonatal Hyperglycemia and Neurodevelopmental Outcomes in Preterm Infants: A Review. Children. 2022; 9(10):1541. https://doi.org/10.3390/children9101541
Chicago/Turabian StyleGuiducci, Silvia, Leonardo Meggiolaro, Anna Righetto, Marco Piccoli, Eugenio Baraldi, and Alfonso Galderisi. 2022. "Neonatal Hyperglycemia and Neurodevelopmental Outcomes in Preterm Infants: A Review" Children 9, no. 10: 1541. https://doi.org/10.3390/children9101541
APA StyleGuiducci, S., Meggiolaro, L., Righetto, A., Piccoli, M., Baraldi, E., & Galderisi, A. (2022). Neonatal Hyperglycemia and Neurodevelopmental Outcomes in Preterm Infants: A Review. Children, 9(10), 1541. https://doi.org/10.3390/children9101541






