Thyroid Function and Metabolic Syndrome in Children and Adolescents with Neuromotor Disability
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Clinical Examination
2.2.2. Biochemical Evaluation
2.2.3. Statistical Analysis
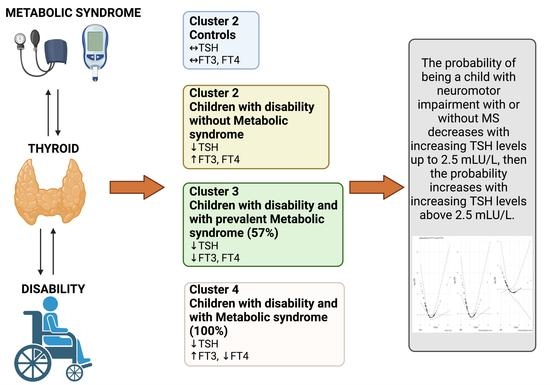
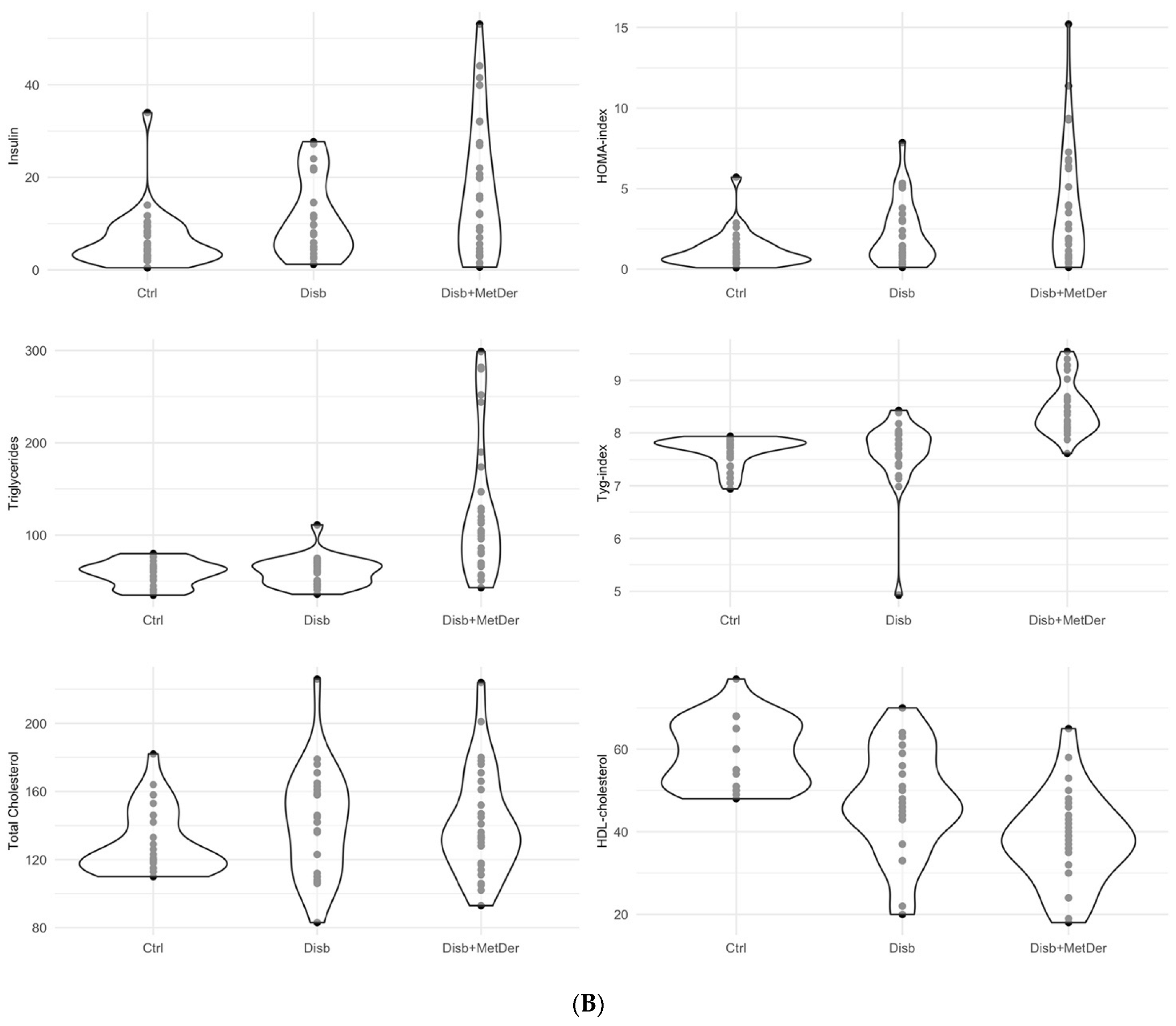
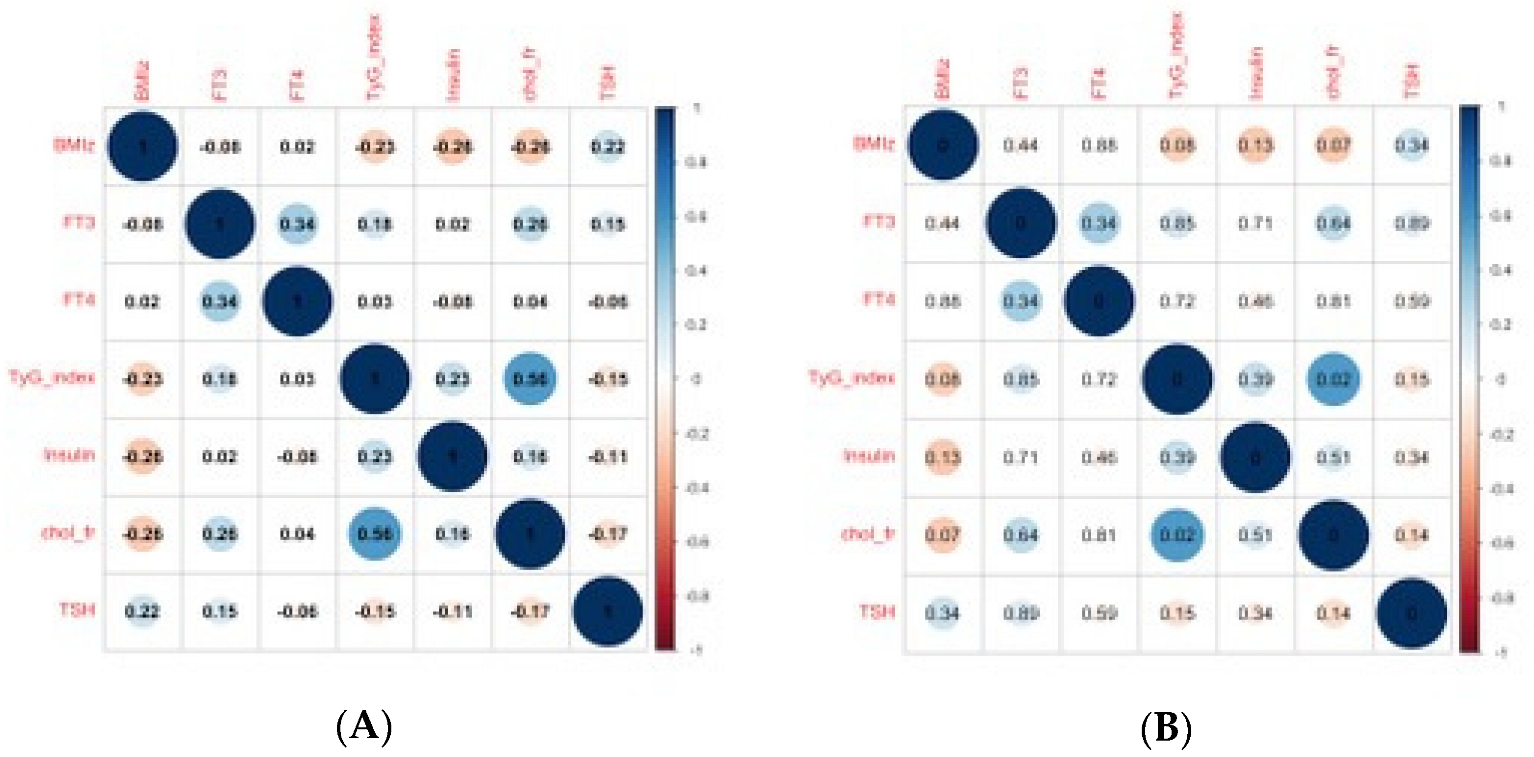
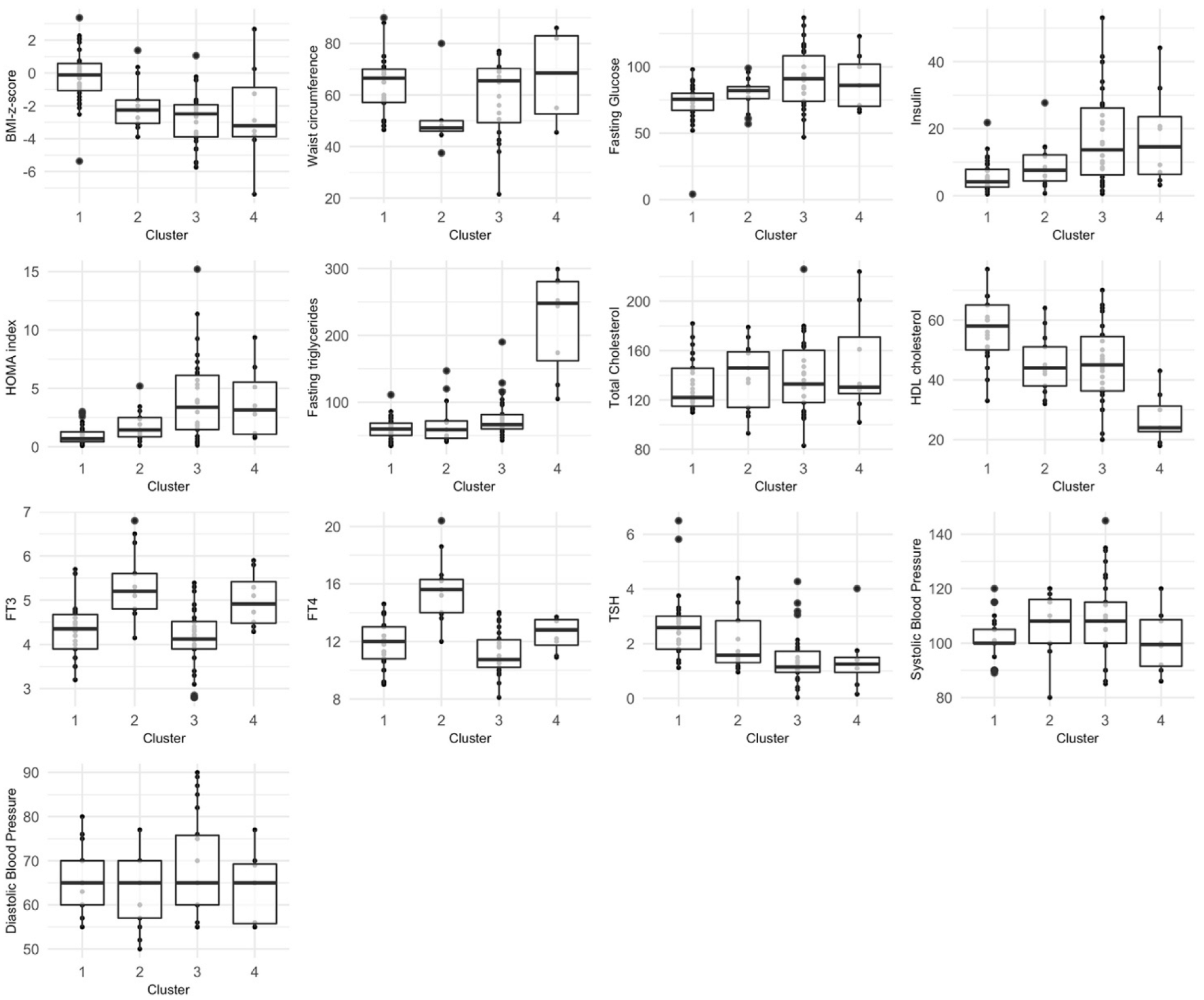
3. Results
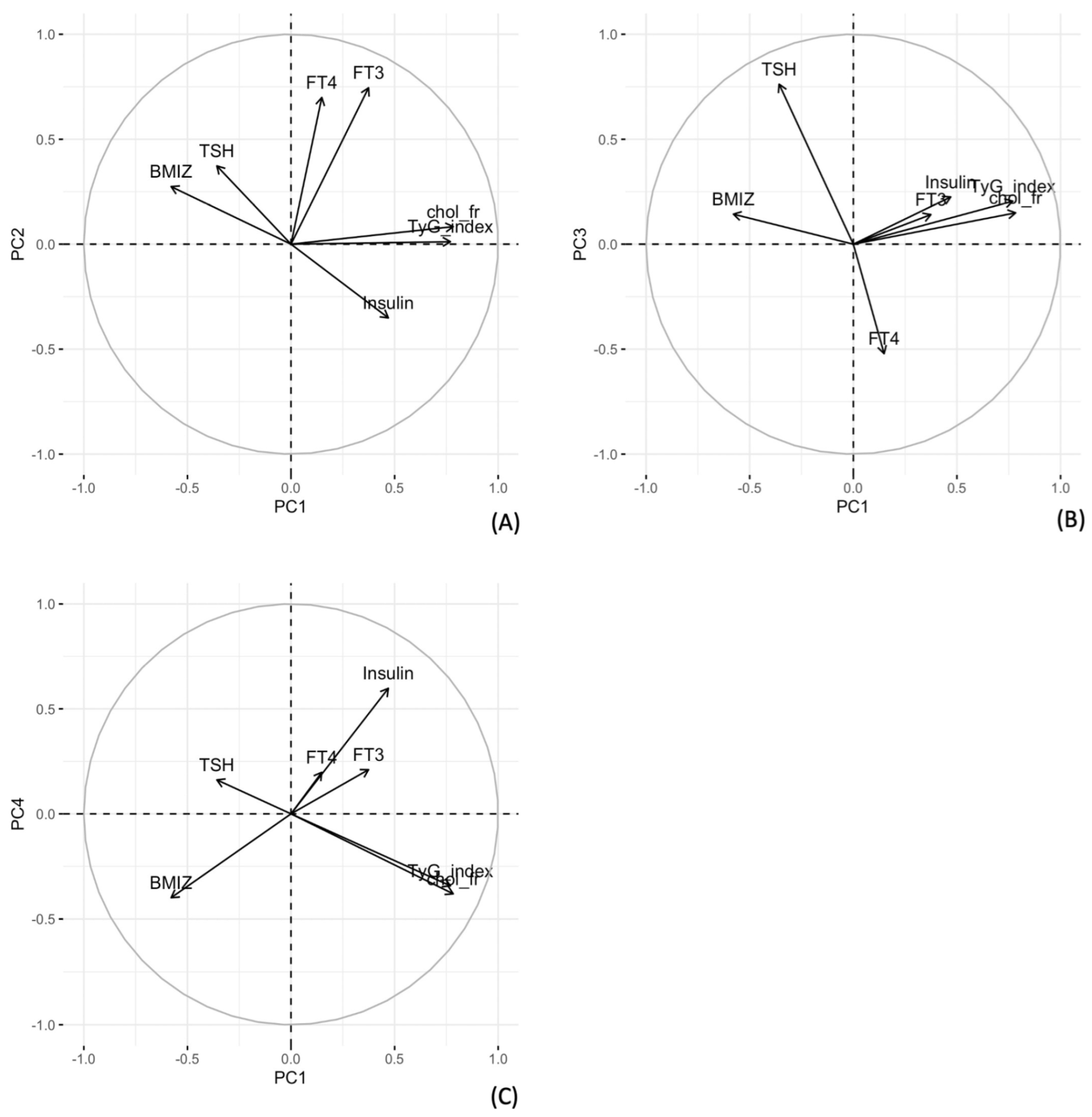
3.1. Principal Component Analysis
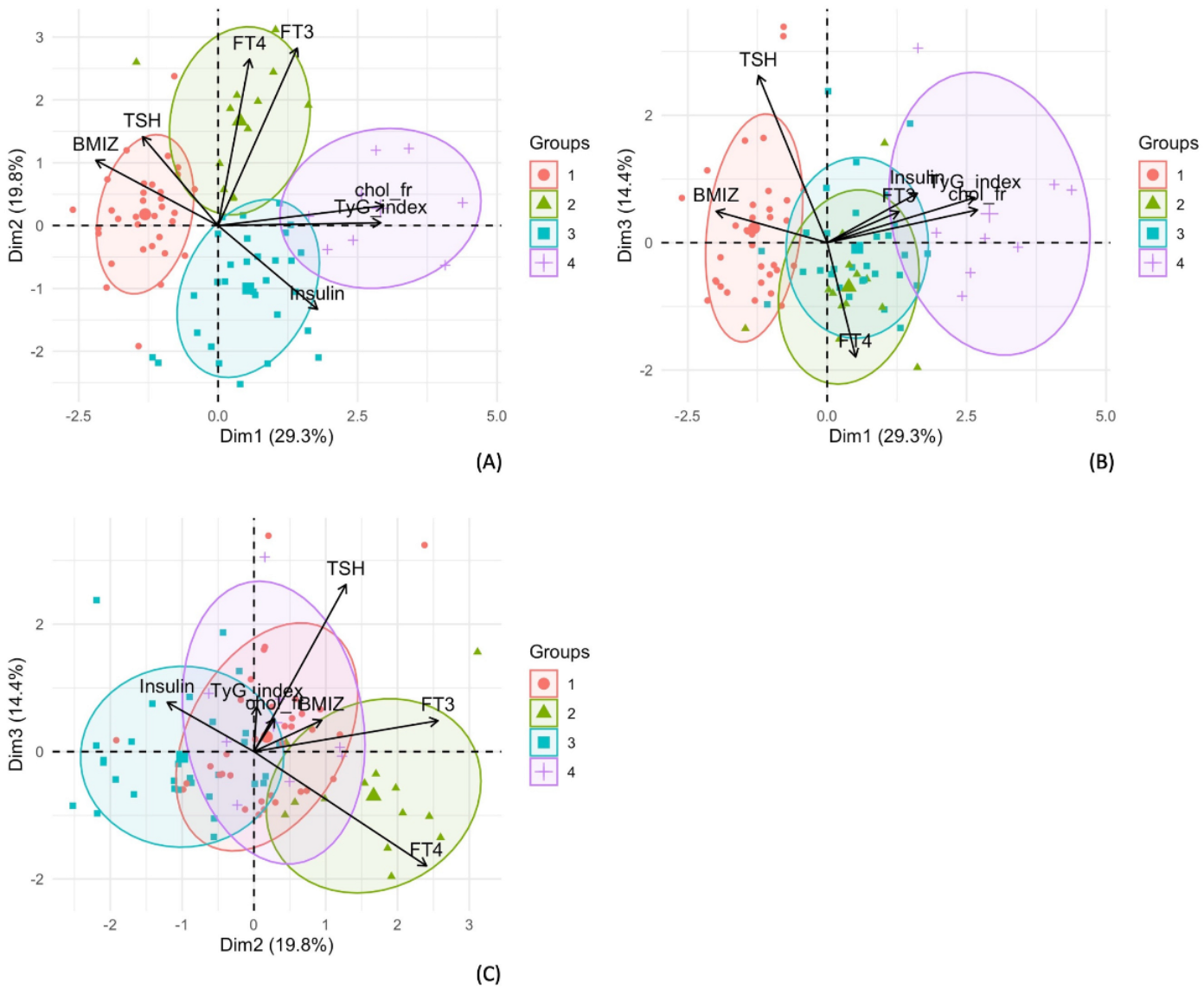
3.2. Hierarchical Clustering on Principal Components
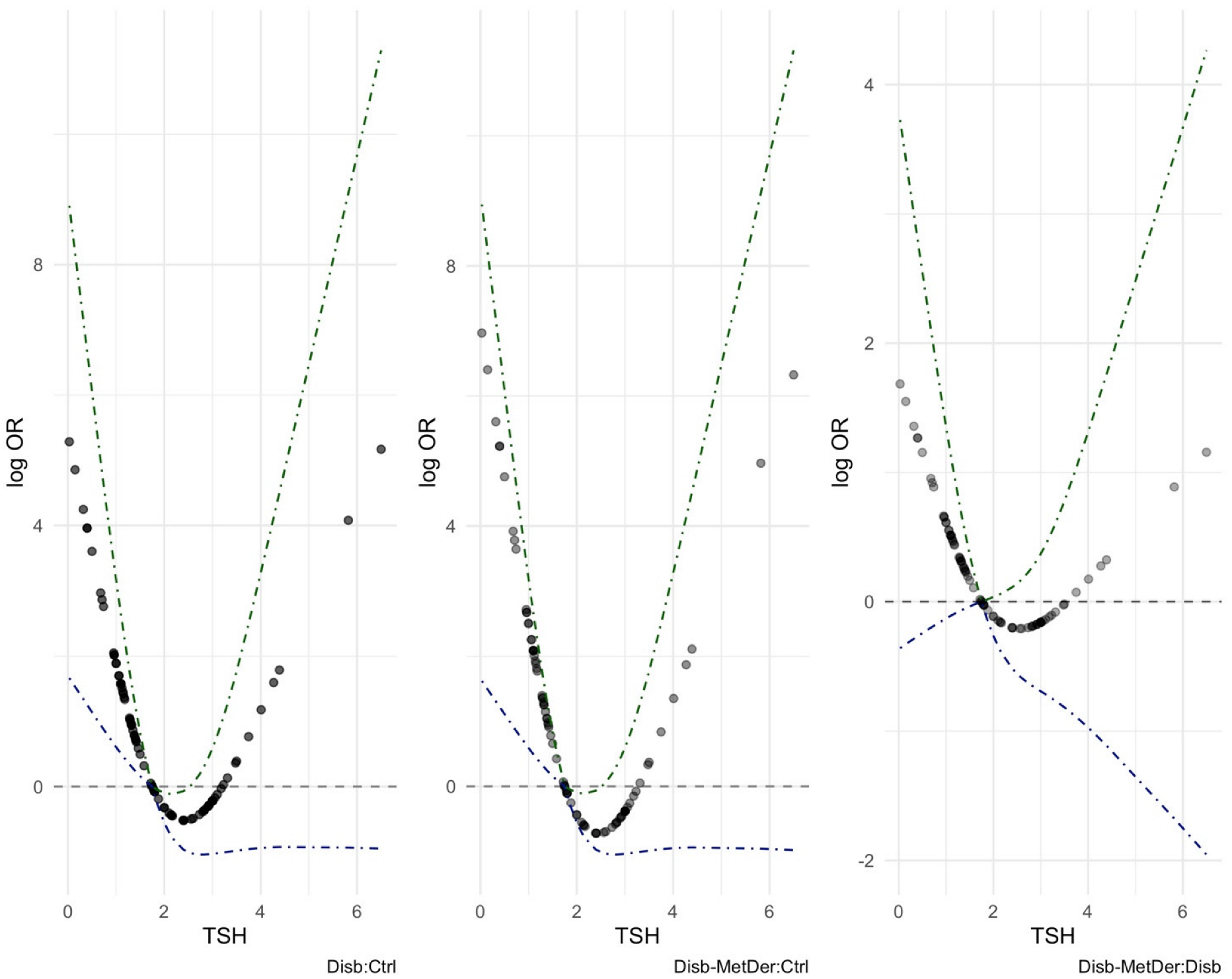
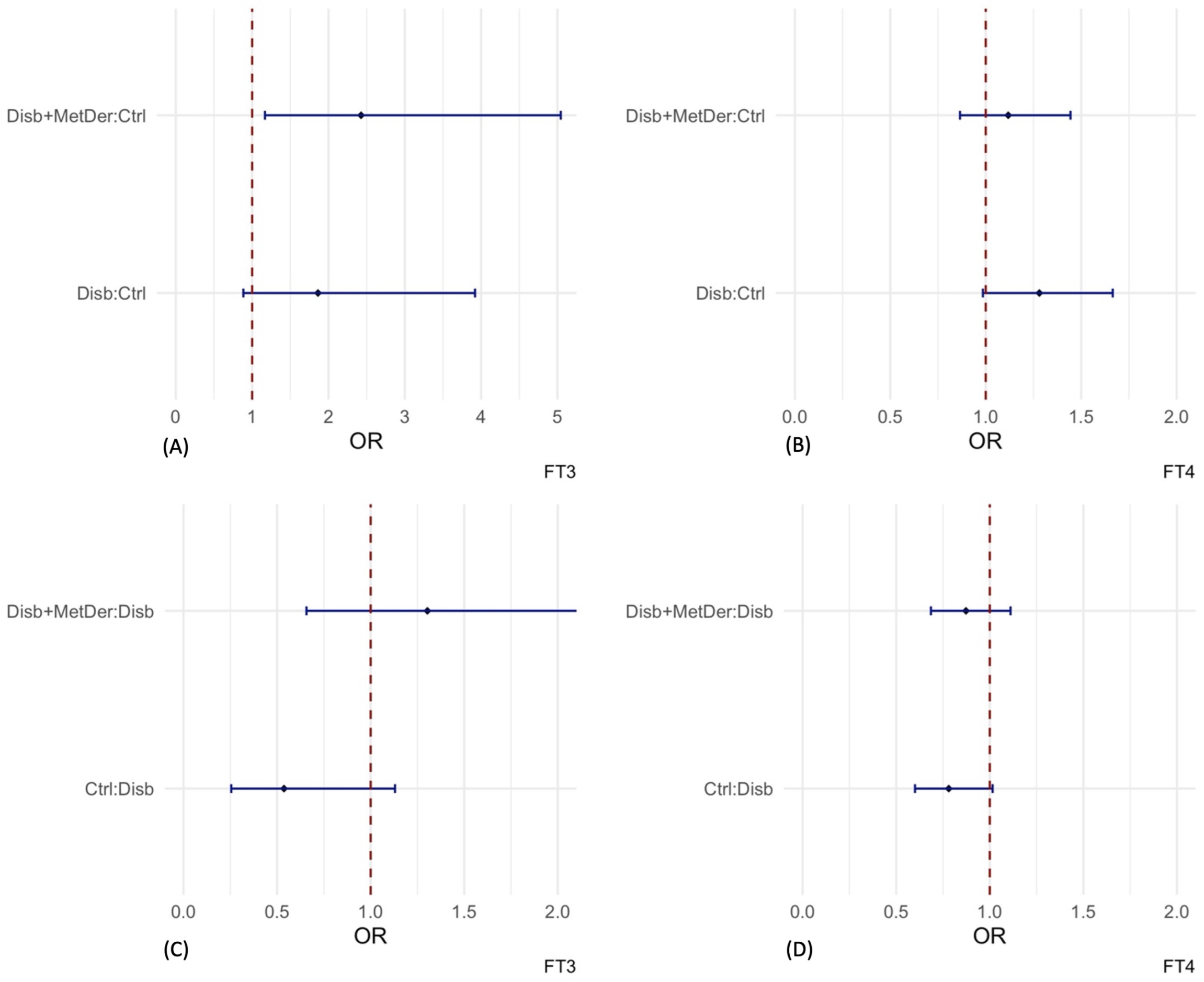
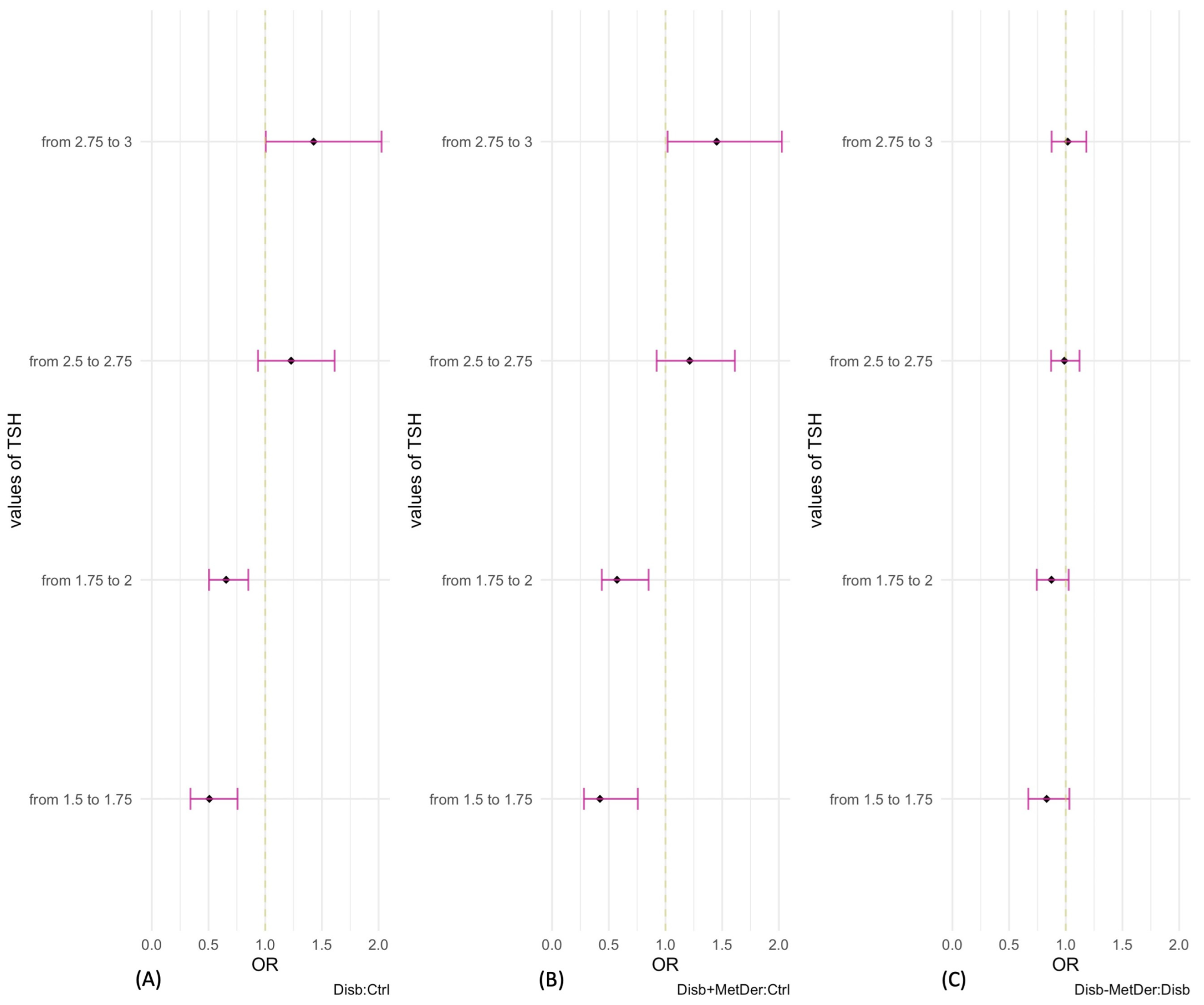
3.3. Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Ahmed, O.M.; El-Gareib, A.W.; El-Bakry, A.M.; Abd El-Tawab, S.M.; Ahmed, R.G. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008, 26, 147–209. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, G.; van Heijningen, S.; Reijne, A.C.; Nyakas, C.; van der Zee, E.A.; Eisel, U.L.M. Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front. Neurosci. 2015, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Guarner-Lans, V.; Rubio-Ruiz, M.E.; Pérez-Torres, I.; Baños de MacCarthy, G. Relation of aging and sex hormones to metabolic syndrome and cardiovascular disease. Exp. Gerontol. 2011, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Tropeano, A.; Corica, D.; Curatola, S.; Li Pomi, A.; Casto, C.; Alibrandi, A.; Pepe, G.; Aversa, T.; Wasniewska, M. The effect of obesity-related allostatic changes on cardio-metabolic risk in euthyroid children. J. Endocrinol. Investig. 2022. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.C.; Rodondi, N.; Harrison, S.; Kanaya, A.M.; Simonsick, E.M.; Miljkovic, I.; Satterfield, S.; Newman, A.B.; Bauer, D.C. Thyroid function and prevalent and incident metabolic syndrome in older adults: The Health, Ageing and Body Composition Study. Clin. Endocrinol. 2012, 76, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.A.; Kim, E.J. Association between thyroid hormone and components of metabolic syndrome in euthyroid Korean adults: A population-based study. Medicine 2021, 100, e28409. [Google Scholar] [CrossRef]
- Tropeano, A.; Corica, D.; Li Pomi, A.; Pepe, G.; Morabito, L.A.; Curatola, S.L.; Casto, C.; Aversa, T.; Wasniewska, M. The metabolic syndrome in pediatrics: Do we have a reliable definition? A systematic review. Eur. J. Endocrinol. 2021, 185, 265–278. [Google Scholar] [CrossRef]
- Calcaterra, V.; Biganzoli, G.; Pelizzo, G.; Cena, H.; Rizzuto, A.; Penagini, F.; Verduci, E.; Bosetti, A.; Lucini, D.; Biganzoli, E.; et al. A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents. Children 2021, 8, 186. [Google Scholar] [CrossRef]
- Koulouri, O.; Moran, C.; Halsall, D.; Chatterjee, K.; Gurnell, M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 745–762. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrou, P.; Maratou, E.; Raptis, A.E.; Tountas, N.; Raptis, S.A.; Dimitriadis, G. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine 2011, 39, 28–32. [Google Scholar] [CrossRef]
- Chen, X.; Deng, S.; Sena, C.; Zhou, C.; Thaker, V. V Relationship of TSH Levels with Cardiometabolic Risk Factors in US Youth and Reference Percentiles for Thyroid Function. J. Clin. Endocrinol. Metab. 2021, 106, e1221–e1230. [Google Scholar] [CrossRef] [PubMed]
- de Moura Souza, A.; Sichieri, R. Association between serum TSH concentration within the normal range and adiposity. Eur. J. Endocrinol. 2011, 165, 11–15. [Google Scholar] [CrossRef]
- Calcaterra, V.; Biganzoli, G.; Ferraro, S.; Verduci, E.; Rossi, V.; Vizzuso, S.; Bosetti, A.; Borsani, B.; Biganzoli, E.; Zuccotti, G. A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome. J. Clin. Med. 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 10 July 2022).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Pelizzo, G.; Calcaterra, V.; Carlini, V.; Fusillo, M.; Manuelli, M.; Klersy, C.; Pasqua, N.; Luka, E.; Albertini, R.; De Amici, M.; et al. Nutritional status and metabolic profile in neurologically impaired pediatric surgical patients. J. Pediatr. Endocrinol. Metab. 2017, 30, 289–300. [Google Scholar] [CrossRef]
- Videira-Silva, A.; Fonseca, H. The effect of a physical activity consultation on body mass index z-score of overweight adolescents: Results from a pediatric outpatient obesity clinic. Eur. J. Pediatr. 2017, 176, 655–660. [Google Scholar] [CrossRef]
- d’Annunzio, G.; Vanelli, M.; Pistorio, A.; Minuto, N.; Bergamino, L.; Iafusco, D.; Lorini, R. Insulin resistance and secretion indexes in healthy Italian children and adolescents: A multicentre study. Acta Biomed. 2009, 80, 21–28. [Google Scholar]
- D’Intino, G.; Lorenzini, L.; Fernandez, M.; Taglioni, A.; Perretta, G.; Del Vecchio, G.; Villoslada, P.; Giardino, L.; Calzà, L. Triiodothyronine administration ameliorates the demyelination/remyelination ratio in a non-human primate model of multiple sclerosis by correcting tissue hypothyroidism. J. Neuroendocrinol. 2011, 23, 778–790. [Google Scholar] [CrossRef]
- Reier, P.J.; Hughes, A.F. An effect of neonatal radiothyroidectomy upon nonmyelinated axons and associated Schwann cells during maturation of the mouse sciatic nerve. Brain Res. 1972, 41, 263–282. [Google Scholar] [CrossRef]
- Franklin, R.J.; Gilson, J.M.; Franceschini, I.A.; Barnett, S.C. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia 1996, 17, 217–224. [Google Scholar] [CrossRef]
- Barakat-Walter, I.; Kraftsik, R. Stimulating effect of thyroid hormones in peripheral nerve regeneration: Research history and future direction toward clinical therapy. Neural Regen. Res. 2018, 13, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.D.F.D.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef] [PubMed]
- Chatzitomaris, A.; Hoermann, R.; Midgley, J.E.; Hering, S.; Urban, A.; Dietrich, B.; Abood, A.; Klein, H.H.; Dietrich, J.W. Thyroid Allostasis-Adaptive Responses of Thyrotropic Feedback Control to Conditions of Strain, Stress, and Developmental Programming. Front. Endocrinol. 2017, 8, 163. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; De Silvestri, A.; Albertini, R.; De Amici, M.; Valenza, M.; Pelizzo, G. Stress Measured by Allostatic Load in Neurologically Impaired Children: The Importance of Nutritional Status. Horm. Res. Paediatr. 2017, 88, 224–230. [Google Scholar] [CrossRef]
- Lennartsson, A.-K.; Jonsdottir, I.H. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology 2011, 36, 1530–1539. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, L.; Fu, J.; An, Y.; Meng, H.; Wang, G. Increased Prolactin is an Adaptive Response to Protect Against Metabolic Disorders in Obesity. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021, 27, 728–735. [Google Scholar] [CrossRef]
- Courtin, F.; Zrouri, H.; Lamirand, A.; Li, W.W.; Mercier, G.; Schumacher, M.; Le Goascogne, C.; Pierre, M. Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid 2005, 15, 931–942. [Google Scholar] [CrossRef]
- Berenberg, R.A.; Forman, D.S.; Wood, D.K.; DeSilva, A.; Demaree, J. Recovery of peripheral nerve function after axotomy: Effect of triiodothyronine. Exp. Neurol. 1977, 57, 349–363. [Google Scholar] [CrossRef]
- Barakat-Walter, I.; Duc, C.; Puymirat, J. Changes in nuclear 3,5,3’-triiodothyronine receptor expression in rat dorsal root ganglia and sciatic nerve during development: Comparison with regeneration. Eur. J. Neurosci. 1993, 5, 319–326. [Google Scholar] [CrossRef] [PubMed]


| Controls | Patients with Neuromotor Impairment | ||
|---|---|---|---|
| Without Metabolic Syndrome | With Metabolic Syndrome | ||
| n | 30 | 25 | 30 |
| Age, years (median (IQR)) | 12.30 (9.15, 13.96) | 8.15 (3.75, 12.54) | 12.39 (4.17, 15.79) |
| BMI sds z-score, (median (IQR)) | −0.17 (−1.18, 0.54) | −1.76 (−2.43, −0.32) | −2.94 (−4.04, −2.30) |
| Sex, (n (%)) | 20 M (67%) | 16 M (64%) | 20 M (67%) |
| Puberal Stage, (n (%)) 1 | 10 (33%) | 16 (64%) | 15 (50%) |
| 2 | 13 (43%) | 4 (16%) | 3 (10%) |
| 3 | 7 (23%) | 5 (20%) | 12 (40%) |
| Fasting blood glucose, mg/dL (median (IQR)) | 74.00 (68.00, 80.00) | 80.00 (71.00, 85.00) | 92.00 (74.00, 106.00) |
| Insulin, µU/mL (median (IQR)) | 1.47 (1.05, 2.20) | 2.08 (1.48, 2.68) | 2.49 (1.40, 3.24) |
| Tryglicerides, mg/dL (median, (IQR)) | 60.50 (51.00, 66.75) | 61.00 (47.00, 70.00) | 103.00 (68.50, 142.50) |
| FT3, pmol/L (median (IQR)) | 4.35 (3.70, 4.68) | 4.46 (4.08, 4.80) | 4.50 (4.11, 5.27) |
| FT4, pmol/L (median (IQR)) | 12.00 (10.72, 13.07) | 12.40 (10.60, 14.00) | 11.65 (10.72, 13.50) |
| TSH, mIU/L (median (IQR)) | 2.40 (1.49, 2.98) | 1.72 (1.06, 2.73) | 1.27 (0.96, 2.10) |
| Cholesterol-HDL, mg/dL (median (IQR)) | 60.00 (51.00, 65.00) | 46.00 (43.00, 56.00) | 37.50 (30.50, 43.75) |
| Cholesterol-total, mg/dL (median (IQR)) | 120.00 (115.00, 145.00) | 142.00 (112.00, 161.00) | 133.00 (120.50, 158.75) |
| HOMA-IR (median (IQR)) | 0.80 (0.47, 1.52) | 1.44 (0.84, 3.06) | 2.65 (0.86, 6.35) |
| Tryg-index (median (IQR)) | 7.76 (7.57, 7.86) | 7.77 (7.55, 7.99) | 8.38 (8.11, 8.64) |
| Systolic blood pressure, mmHg (median (IQR)) | 100.00 (100.00, 105.00) | 108.00 (100.00, 115.00) | 100.00 (99.00, 118.50) |
| Diastolic blood pressure, mmHg (median (IQR)) | 65.00 (60.00, 70.00) | 65.00 (60.00, 70.00) | 65.00 (56.00, 75.75) |
| Variables | Cerebral Damage | Neuromuscular Diseases |
|---|---|---|
| n | 38 | 17 |
| Fasting blood glucose, mg/dL (median (IQR)) | 85.00 (68.00, 100.00) | 85.00 (76.00, 93.00) |
| Insulin, µU/mL (median (IQR)) | 2.21 (1.39, 3.09) | 2.47 (1.77, 3.00) |
| Tryglicerides, mg/dL (median, (IQR)) | 72.00 (56.25, 109.50) | 70.00 (47.00, 102.00) |
| FT3, pmol/L (median (IQR)) | 4.21 (3.98, 4.70) | 5.30 (4.70, 5.60) |
| FT4, pmol/L (median (IQR)) | 11.35 (10.50, 13.47) | 13.50 (12.00, 15.60) |
| TSH mUI/L (median (IQR)) | 1.44 (0.95, 2.97) | 1.41 (1.10, 1.74) |
| Cholesterol-HDL, mg/dL (median (IQR)) | 40.50 (33.50, 47.00) | 44.00 (35.00, 54.00) |
| Cholesterol-total, mg/dL (median (IQR)) | 133.00 (114.75, 160.25) | 146.00 (133.00, 161.00) |
| HOMA-IR (median (IQR)) | 1.67 (0.79, 5.00) | 2.50 (1.44, 3.51) |
| Tryg-index (median (IQR)) | 8.12 (7.88, 8.43) | 7.97 (7.58, 8.18) |
| Systolic blood pressure, mmHg (median (IQR)) | 108.00 (99.25, 115.75) | 100.00 (100.00, 115.00) |
| Diastolic blood pressure, mmHg (median (IQR)) | 65.00 (57.75, 76.00) | 65.00 (60.00, 65.00) |
| status (%) | ||
| 0 | 0 (0.0) | 0 (0.0) |
| 1 | 14 (36.8) | 11 (64.7) |
| 2 | 24 (63.2) | 6 (35.3) |
| Cluster/Conditions | Control | Disability | Disability + Metabolic Derangement | Total |
|---|---|---|---|---|
| 1 | 79% (27) | 18% (6) | 3% (1) | 100% (34) |
| 2 | 0% (0) | 69% (9) | 31% (4) | 100% (13) |
| 3 | 10% (3) | 33% (10) | 57% (17) | 100% (30) |
| 4 | 0% (0) | 0% (0) | 100% (8) | 100% (8) |
| Total | 35% (30) | 29% (25) | 35% (30) | 100% (85) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Biganzoli, G.; Ferraro, S.; Mari, A.; Mandelli, A.; Fabiano, V.; Carlucci, P.; Pelizzo, G.; Zoia, E.; Lanfranchi, G.; et al. Thyroid Function and Metabolic Syndrome in Children and Adolescents with Neuromotor Disability. Children 2022, 9, 1531. https://doi.org/10.3390/children9101531
Calcaterra V, Biganzoli G, Ferraro S, Mari A, Mandelli A, Fabiano V, Carlucci P, Pelizzo G, Zoia E, Lanfranchi G, et al. Thyroid Function and Metabolic Syndrome in Children and Adolescents with Neuromotor Disability. Children. 2022; 9(10):1531. https://doi.org/10.3390/children9101531
Chicago/Turabian StyleCalcaterra, Valeria, Giacomo Biganzoli, Simona Ferraro, Alessandra Mari, Anna Mandelli, Valentina Fabiano, Patrizia Carlucci, Gloria Pelizzo, Elena Zoia, Giulia Lanfranchi, and et al. 2022. "Thyroid Function and Metabolic Syndrome in Children and Adolescents with Neuromotor Disability" Children 9, no. 10: 1531. https://doi.org/10.3390/children9101531
APA StyleCalcaterra, V., Biganzoli, G., Ferraro, S., Mari, A., Mandelli, A., Fabiano, V., Carlucci, P., Pelizzo, G., Zoia, E., Lanfranchi, G., Castaldi, S., Boracchi, P., Biganzoli, E., & Zuccotti, G. (2022). Thyroid Function and Metabolic Syndrome in Children and Adolescents with Neuromotor Disability. Children, 9(10), 1531. https://doi.org/10.3390/children9101531