High Prevalence of Hypovitaminosis D in Adolescents Attending a Reference Centre for the Treatment of Obesity in Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Methods
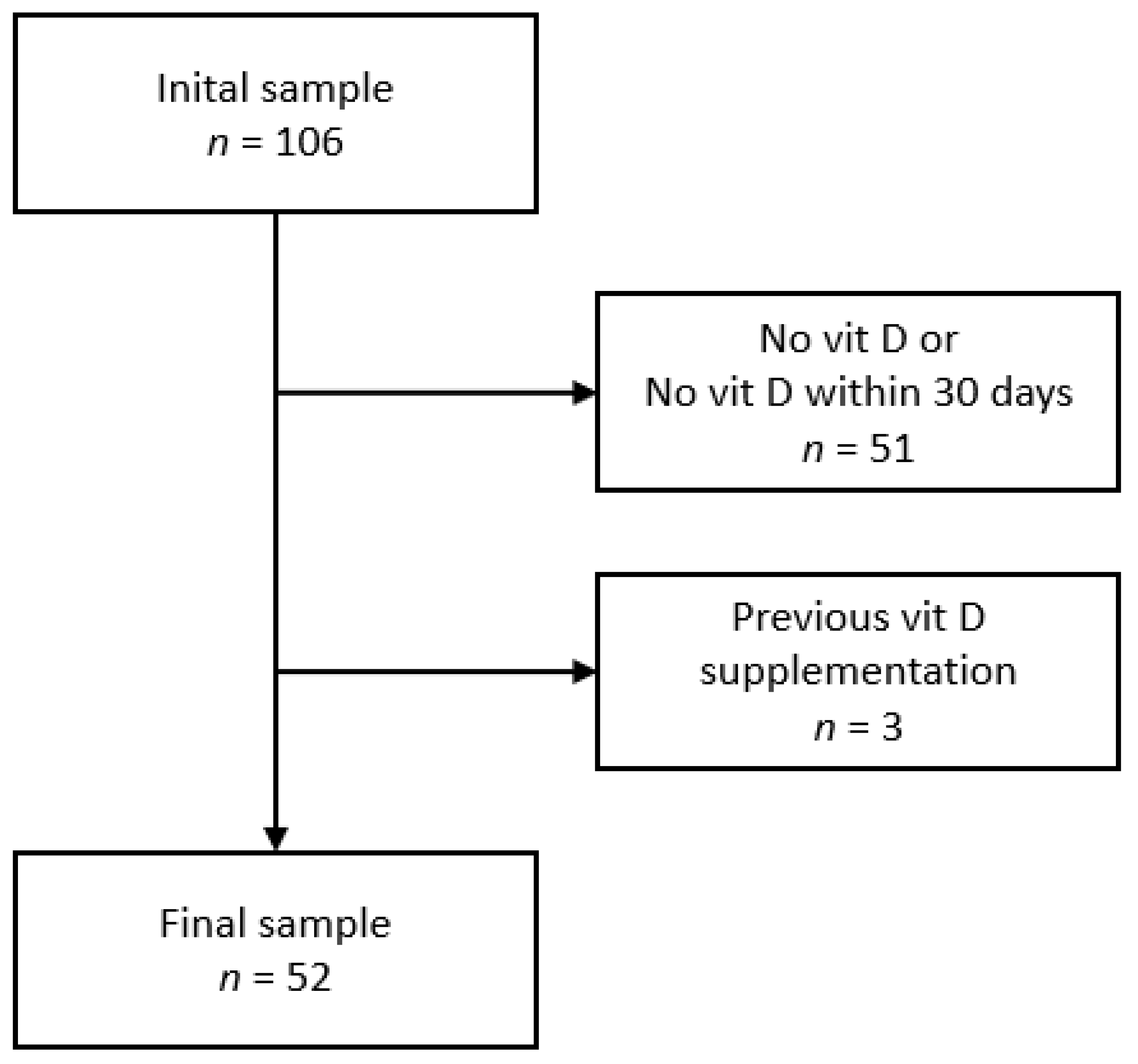
2.3. Inclusion and Exclusion Criteria
2.4. Ethical Statement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Prevalence of Hypovitaminosis D
3.3. Associations between Vitamin D Levels, Obesity Markers and Cardiovascular Risk Factors
4. Discussion
4.1. Associations between Vitamin D Levels and Cardiovascular Risk Factors
4.2. Study Limitations
5. Implications for Practice
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiamenghi, V.I.; de Mello, E.D. de Vitamin D deficiency in children and adolescents with obesity: A meta-analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. (Lausanne) 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, O.; Wilhelm-bals, A. Hypovitaminosis D in migrant children in Switzerland: A retrospective study. Eur. J. Pediatr. 2021, 180, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, K.A.; Magalhães, E.I.D.S.; Loureiro, L.M.R.; Sant’Ana, L.F.D.R.; Ribeiro, A.Q.; Novaes, J.F. De Calcium intake, serum vitamin D and obesity in children: Is there an association? Rev. Paul. Pediatr. 2015, 33, 222–229. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, C.; Shu, Y.; Xie, Z.; Lu, C.; Mo, X. Serum 25-hydroxyVitamin D is associated with obesity and metabolic parameters in US children. Public Health Nutr. 2020, 23, 1214–1222. [Google Scholar] [CrossRef]
- Rafiq, S.; Jeppesen, P. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 59. [Google Scholar] [CrossRef]
- Wojcik, M.; Janus, D.; Kalicka-Kasperczyk, A.; Sztefko, K.; Starzyk, J.B. The potential impact of the hypovitaminosis d on metabolic complications in obese adolescents—Preliminary results. Ann. Agric. Environ. Med. 2017, 24, 636–639. [Google Scholar] [CrossRef]
- Kelishadi, R.; Farajzadegan, Z.; Bahreynian, M. Association between vitamin D status and lipid profile in children and adolescents: A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2014, 65, 404–410. [Google Scholar] [CrossRef]
- Timothy, G.; Lohmann Alex, F.; Roche, R.M. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- WHO. WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents 2009; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Grossman, Z.; Hadjipanayis, A.; Stiris, T.; del Torso, S.; Mercier, J.C.; Valiulis, A.; Shamir, R. Vitamin D in European children—statement from the European Academy of Paediatrics (EAP). Eur. J. Pediatr. 2017, 176, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Rabufetti, A.; Milani, G.P.; Lava, S.A.G.; Edefonti, V.; Bianchetti, M.G.; Stettbacher, A.; Muggli, F.; Simonetti, G. Vitamin D status among male late adolescents living in Southern Switzerland: Role of body composition and lifestyle. Nutrients 2019, 11, 2727. [Google Scholar] [CrossRef] [PubMed]
- Parel, N.; Bochud, M.; Rezzi, S.; Chatelan, A.; Jotterand Chaparro, C. Vitamin D dietary intake and status in a sample of adolescents. Clin. Nutr. Open Sci. 2022, 43, 56–66. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F.; Chueca-Guindulain, M.J.; Berrade-Zubiri, S.; Moreno-Gónzalez, P.; Malumbres-Chacón, M. Prevalence of hypovitaminosis D and associated factors in Spanish population of school children and adolescents. Aten. Primaria 2018, 50, 422–429. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F.; Chueca-Guindulain, M.J.; Berrade-Zubiri, S. Prevalence of hypovitaminosis D and associated factors in obese Spanish children. Nutr. Diabetes 2017, 7, e248. [Google Scholar] [CrossRef]
- Vierucci, F.; Del Pistoia, M.; Fanos, M.; Erba, P.; Saggese, G. Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital. J. Pediatr. 2014, 40, 54. [Google Scholar] [CrossRef]
- Plesner, J.L.; Dahl, M.; Fonvig, C.E.; Nielsen, T.R.H.; Kloppenborg, J.T.; Pedersen, O.; Hansen, T.; Holm, J.-C. Obesity is associated with vitamin D deficiency in Danish children and adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Lambrinou, C.-P.; Tsoutsoulopoulou, K.; Binou, P.; Karachaliou, A.; Breidenassel, C.; Gonzalez-Gross, M.; Kiely, M.; Cashman, K.D. A systematic review of vitamin D status in southern European countries. Eur. J. Nutr. 2018, 57, 2001–2036. [Google Scholar] [CrossRef]
- Religi, A.; Backes, C.; Chatelan, A.; Bulliard, J.L.; Vuilleumier, L.; Moccozet, L.; Bochud, M.; Vernez, D. Estimation of exposure durations for vitamin D production and sunburn risk in Switzerland. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 742–752. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Chattranukulchai Shantavasinkul, P.; Nimitphong, H. Vitamin D and Visceral Obesity in Humans: What Should Clinicians Know? Nutrients 2022, 14, 3075. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gangloff, A.; Bergeron, J.; Lemieux, I.; Després, J.-P. Changes in circulating vitamin D levels with loss of adipose tissue. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kishore, S. Role of parathyroid hormone in determination of fat mass in patients with Vitamin D deficiency. Indian J. Endocrinol. Metab. 2017, 21, 848–853. [Google Scholar] [CrossRef]
- Karava, V.; Kondou, A.; Dotis, J.; Christoforidis, A.; Taparkou, A.; Tsioni, K.; Farmaki, E.; Kollios, K.; Siomou, E.; Liakopoulos, V.; et al. Association Between Secondary Hyperparathyroidism and Body Composition in Pediatric Patients With Moderate and Advanced Chronic Kidney Disease. Front. Pediatr. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Stanley, T.; Bredella, M.A.; Pierce, L.; Misra, M. The ratio of parathyroid hormone to vitamin D is a determinant of cardiovascular risk and insulin sensitivity in adolescent girls. Metab. Syndr. Relat. Disord. 2013, 11, 56–62. [Google Scholar] [CrossRef]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Shab-bidar, C.L.W.S. Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef]
- Dix, C.F.; Barcley, J.L.; Wright, O.R.L. The role of vitamin D in adipogenesis. Nutr. Rev. 2018, 76, 47–59. [Google Scholar] [CrossRef]
- Courraud, J.; Quist, J.S.; Kontopodi, E.; Jensen, M.B.; Bjerrum, P.J.; Helge, J.W.; Sorensen, K. Dietary habits, metabolic health and Vitamin D status in Greenlandic children. Public Health Nutr. 2020, 23, 904–913. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- De la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A.G. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J. Clin. Endocrinol. Metab. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
| Girls | Boys | p-Value | |
|---|---|---|---|
| Sample size | 16 | 36 | |
| Age (years) | 14.0 ± 2.0 | 13.1 ± 2.2 | 0.170 |
| Swiss (%) | 12 (75.0) | 11 (30.6) | 0.006 |
| Height (cm) | 161 ± 10 | 160 ± 12 | 0.868 |
| Height (z-score) | 0.6 ± 1.3 | 0.7 ± 1.3 | 0.800 |
| Weight (kg) | 87.3 ± 18.4 | 74.8 ± 16.4 | 0.018 |
| Body mass index (kg/m2) | 33.4 ± 5.1 | 28.8 ± 3.7 | <0.001 |
| Body mass index z-score | 2.9 ± 0.5 | 2.6 ± 0.5 | 0.131 |
| Body mass index categories (%) | 0.111 | ||
| Obesity | 8 (50.0) | 27 (75.0) | |
| Severe obesity * | 8 (50.0) | 9 (25.0) | |
| Vitamin D level (ng/mL) | 16.9 [9.7–26.3] | 19.2 [12.3–24.0] | 0.620 § |
| Hypovitaminosis D (%) | 14 (87.5) | 32 (88.9) | 1.000 |
| Sample Size | Correlation | p-Value | |
|---|---|---|---|
| Age (years) | 52 | −0.290 (−0.556; −0.017) | 0.029 |
| Body mass index (kg/m2) | 52 | −0.286 (−0.572; −0.001) | 0.037 |
| Body mass index z-score | 52 | −0.052 (−0.326; 0.223) | 0.713 |
| Parathormone | 35 | −0.353 (−0.675; −0.030) | 0.032 |
| Calcium (mmol/L) | 32 | 0.385 (0.070; 0.699) | 0.018 |
| Total cholesterol (mmol/L) | 49 | 0.104 (−0.186; 0.395) | 0.490 |
| LDL cholesterol (mmol/L) | 49 | −0.068 (−0.361; 0.224) | 0.651 |
| HDL cholesterol (mmol/L) | 49 | 0.224 (−0.051; 0.500) | 0.092 |
| Triglycerides (mmol/L) | 49 | 0.233 (−0.059; 0.525) | 0.128 |
| Fasting Glucose (mmol/L) | 30 | 0.129 (−0.290; 0.548) | 0.546 |
| Insulin | 10 | −0.036 (−0.849; 0.776) | 0.931 |
| Systolic blood pressure (percentile) | 31 | −0.190 (−0.589; 0.209) | 0.351 |
| Diastolic blood pressure (percentile) | 31 | −0.297 (−0.692; 0.097) | 0.139 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patriota, P.; Borloz, S.; Ruiz, I.; Bouthors, T.; Rezzi, S.; Marques-Vidal, P.; Hauschild, M. High Prevalence of Hypovitaminosis D in Adolescents Attending a Reference Centre for the Treatment of Obesity in Switzerland. Children 2022, 9, 1527. https://doi.org/10.3390/children9101527
Patriota P, Borloz S, Ruiz I, Bouthors T, Rezzi S, Marques-Vidal P, Hauschild M. High Prevalence of Hypovitaminosis D in Adolescents Attending a Reference Centre for the Treatment of Obesity in Switzerland. Children. 2022; 9(10):1527. https://doi.org/10.3390/children9101527
Chicago/Turabian StylePatriota, Pollyanna, Sylvie Borloz, Inge Ruiz, Thérèse Bouthors, Serge Rezzi, Pedro Marques-Vidal, and Michael Hauschild. 2022. "High Prevalence of Hypovitaminosis D in Adolescents Attending a Reference Centre for the Treatment of Obesity in Switzerland" Children 9, no. 10: 1527. https://doi.org/10.3390/children9101527
APA StylePatriota, P., Borloz, S., Ruiz, I., Bouthors, T., Rezzi, S., Marques-Vidal, P., & Hauschild, M. (2022). High Prevalence of Hypovitaminosis D in Adolescents Attending a Reference Centre for the Treatment of Obesity in Switzerland. Children, 9(10), 1527. https://doi.org/10.3390/children9101527






