Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study
Abstract
1. Introduction
2. Methods
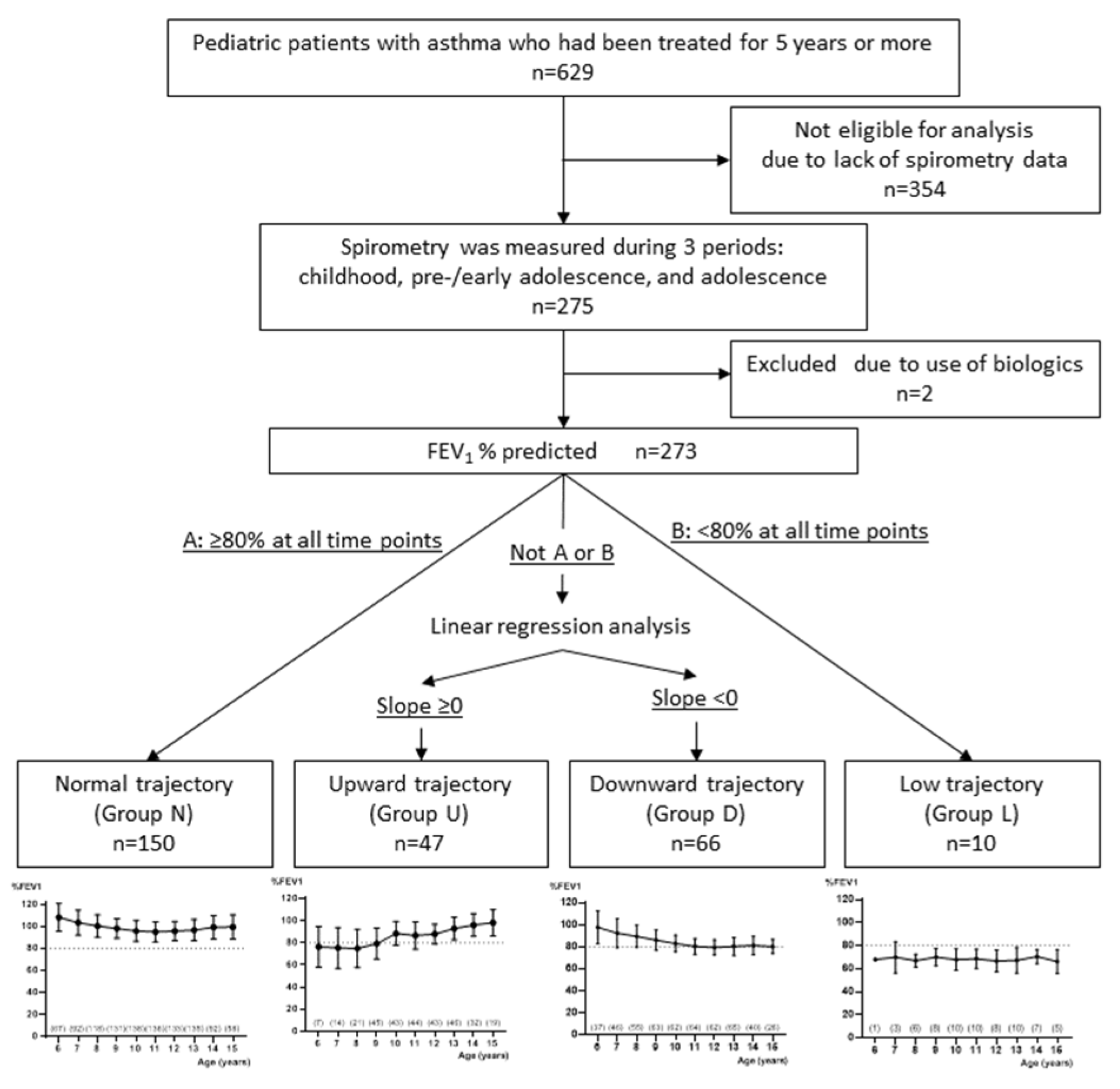
2.1. The Study Population
2.2. Data Collection
2.3. Lung Function
2.4. Statistical Analyses
2.5. Ethics
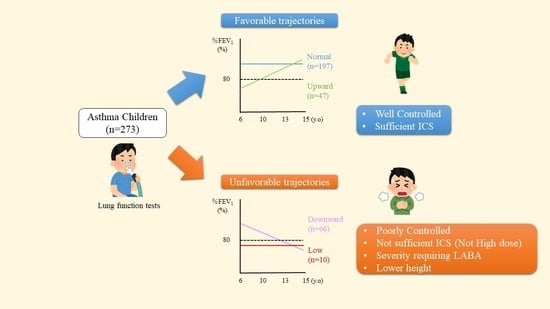
3. Results
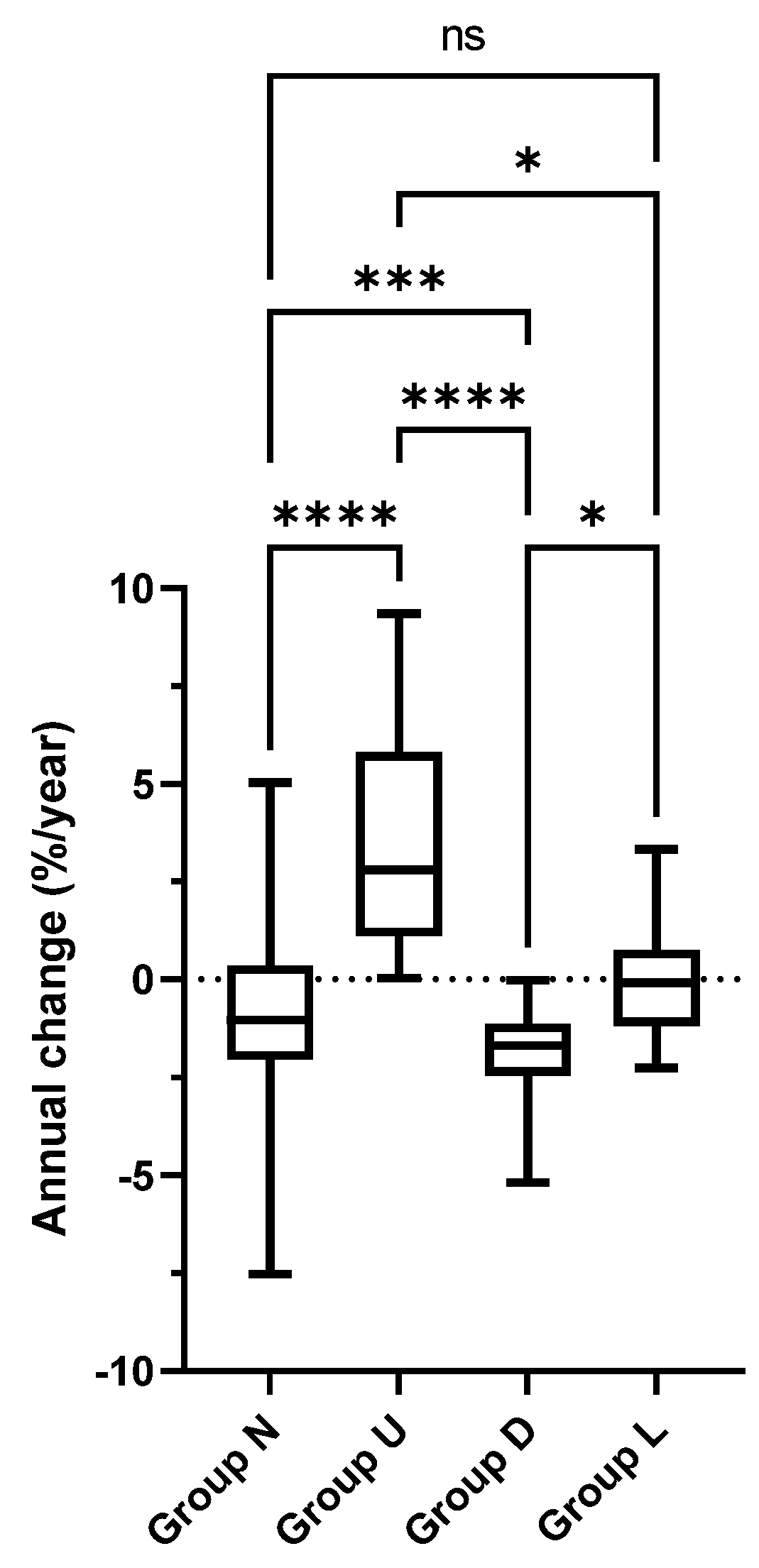
3.1. Classification of %FEV1 Trajectories
3.2. Clinical Characteristics of the Subjects
3.3. Factors Associated with Unfavorable Lung Function Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lange, P.; Celli, B.; Agusti, A.; Jensen, B.G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.C.; Castaldi, P.J.; Cho, M.H.; Hersh, C.P.; Rahaghi, F.N.; Sanchez-Ferrero, G.V.; Parker, M.M.; Litonjua, A.A.; Sparrow, D.; Dy, J.G.; et al. Longitudinal modeling of lung function trajectories in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.S.; Lodge, C.J.; Perret, J.L.; Lowe, A.; Hamilton, G.S.; Thompson, B.; Giles, G.; Tan, D.; Erbas, B.; Pirkis, J.; et al. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: A prospective cohort study. Lancet Respir. Med. 2021, 9, 387–396. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Sadasivam, S.; Mamun, A.A.; Najman, J.M.; Williams, G.M.; O’Callaghan, M.J. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax 2009, 64, 810–814. [Google Scholar] [CrossRef]
- Gross, S.J.; Iannuzzi, D.M.; Kveselis, D.A.; Anbar, R.D. Effect of preterm birth on pulmonary function at school age: A prospective controlled study. J. Pediatr. 1998, 133, 188–192. [Google Scholar] [CrossRef]
- Gibson, A.M.; Reddington, C.; McBride, L.; Callanan, C.; Robertson, C.; Doyle, L.W. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr. Pulmonol. 2015, 50, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.C.; Sheils, C.A.; Gaffin, J.M.; Hersh, C.P.; Rhein, L.M.; Hayden, L.P. Lung function trajectories in children with post-prematurity respiratory disease: Identifying risk factors for abnormal growth. Respir. Res. 2021, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Andersson, S.; Bush, A.; Cheong, J.L.Y.; Clemm, H.; Evensen, K.A.I.; Gough, A.; Halvorsen, T.; Hovi, P.; Kajantie, E.; et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: A meta-analysis of individual participant data. Lancet Respir. Med. 2019, 7, 677–686. [Google Scholar] [CrossRef]
- Dezateux, C.A.; Stocks, J.; Dundas, I.; Jackson, E.A.; Fletcher, M.E. The relationship between tPTEF:tE and specific airway conductance in infancy. Pediatr. Pulmonol. 1994, 18, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kitcharoensakkul, M.; Bacharier, L.B.; Schweiger, T.L.; Wilson, B.; Goss, C.W.; Lew, D.; Schechtman, K.B.; Castro, M. Lung function trajectories and bronchial hyperresponsiveness during childhood following severe RSV bronchiolitis in infancy. Pediatr. Allergy Immunol. 2021, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, F.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Greene, J.M.; Herbison, G.P.; Sears, M.R. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: A longitudinal population study from childhood to adulthood. Am. J. Respir. Crit. Care Med. 2002, 165, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Koefoed, H.J.L.; Zwitserloot, A.M.; Vonk, J.M.; Koppelman, G.H. Asthma, bronchial hyperresponsiveness, allergy and lung function development until early adulthood: A systematic literature review. Pediatr. Allergy Immunol. 2021, 32, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Childhood Asthma Management Program Research Group; Szefler, S.; Weiss, S.; Tonascia, J.; Adkinson, N.F.; Bender, B.; Cherniack, R.; Donithan, M.; Kelly, H.W.; Reisman, J.; et al. Long-term effects of budesonide or nedocromil in children with asthma. N. Engl. J. Med. 2000, 343, 1054–1063. [Google Scholar] [PubMed]
- Covar, R.A.; Spahn, J.D.; Murphy, J.R.; Szefler, S.J. Progression of asthma measured by lung function in the childhood asthma management program. Am. J. Respir. Crit. Care Med. 2004, 170, 234–241. [Google Scholar] [CrossRef]
- Grol, M.H.; Gerritsen, J.; Vonk, J.M.; Schouten, J.P.; Koeter, G.H.; Rijcken, B.; Postma, D.S. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years. A 30-year follow-up study. Am. J. Respir. Crit. Care Med. 1999, 160, 1830–1837. [Google Scholar] [CrossRef]
- de Marco, R.; Marcon, A.; Jarvis, D.; Accordini, S.; Bugiani, M.; Cazzoletti, L.; Cerveri, I.; Corsico, A.; Gislason, D.; Gulsvik, A.; et al. Inhaled steroids are associated with reduced lung function decline in subjects with asthma with elevated total IgE. J. Allergy Clin. Immunol. 2007, 119, 611–617. [Google Scholar] [CrossRef]
- Liu, A.H.; Zeiger, R.; Sorkness, C.; Mahr, T.; Ostrom, N.; Burgess, S.; Rosenzweig, J.C.; Manjunath, R. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allergy Clin. Immunol. 2007, 119, 817–825. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Takase, M.; Sakata, H.; Shikada, M.; Tatara, K.; Fukushima, T.; Miyakawa, T. Development of reference equations for spirometry in Japanese children aged 6–18 years. Pediatr. Pulmonol. 2013, 48, 35–44. [Google Scholar] [CrossRef]
- Ogata, T.; Tanaka, T.; Kagami, M. Target height and target range for Japanese children: Revisited. Clin. Pediatr. Endocrinol. 2007, 16, 85–87. [Google Scholar] [CrossRef]
- Kato, N.; Takimoto, H.; Sudo, N. The Cubic Functions for Spline Smoothed L, S and M Values for BMI Reference Data of Japanese Children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Nishihara, M.; Kimura, T.; Shiraishi, J.; Hirano, S.; Kitajima, H.; Fujimura, M. Longitudinal impairment of lung function in school-age children with extremely low birth weights. Pediatr. Pulmonol. 2017, 52, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Menezes, A.M.B.; Goncalves, H.; Perez-Padilla, R.; Jarvis, D.; de Oliveira, P.D.; Wehrmeister, F.C. Characterisation of pulmonary function trajectories: Results from a Brazilian cohort. ERJ Open Res. 2020, 6, 65–2020. [Google Scholar] [CrossRef] [PubMed]
- Lodrup, C.K.C.; Mowinckel, P.; Hovland, V.; Haland, G.; Riiser, A.; Carlsen, K.H. Lung function trajectories from birth through puberty reflect asthma phenotypes with allergic comorbidity. J. Allergy Clin. Immunol. 2014, 134, 917–923. [Google Scholar] [CrossRef]
- Belgrave, D.C.M.; Granell, R.; Turner, S.W.; Curtin, J.A.; Buchan, I.E.; Le, S.P.N.; Simpson, A.; Henderson, A.J.; Custovic, A. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: A retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med. 2018, 6, 526–534. [Google Scholar] [CrossRef]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Agusti, A.; Faner, R. Lung function trajectories in health and disease. Lancet Respir. Med. 2019, 7, 358–364. [Google Scholar] [CrossRef]
- Young, S.; Le, S.P.N.; Geelhoed, G.C.; Stick, S.M.; Turner, K.J.; Landau, L.I. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N. Engl. J. Med. 1991, 324, 1168–1173. [Google Scholar] [CrossRef]
- Turner, S.W.; Palmer, L.J.; Rye, P.J.; Gibson, N.A.; Judge, P.K.; Cox, M.; Young, S.; Goldblatt, J.; Landau, L.I.; Le Souëf, P.N. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am. J. Respir. Crit. Care Med. 2004, 169, 921–927. [Google Scholar] [CrossRef]
- Owens, L.; Laing, I.A.; Zhang, G.; Turner, S.; Le Souëf, P.N. Airway function in infancy is linked to airflow measurements and respiratory symptoms from childhood into adulthood. Pediatr. Pulmonol. 2018, 53, 1082–1088. [Google Scholar] [CrossRef]
- Thunqvist, P.; Gustafsson, P.M.; Schultz, E.S.; Bellander, T.; Berggren-Brostrom, E.; Norman, M.; Wickman, M.; Melén, E.; Hallberg, J. Lung Function at 8 and 16 Years After Moderate-to-Late Preterm Birth: A Prospective Cohort Study. Pediatrics 2016, 137, e20152056. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Turkovic, L.; Wilson, A.C.; Verheggen, M.; Logie, K.M.; Pillow, J.J.; Hall, G.L. Lung function trajectories throughout childhood in survivors of very preterm birth: A longitudinal cohort study. Lancet Child Adolesc. Health 2018, 2, 350–359. [Google Scholar] [CrossRef]
- Stern, D.A.; Morgan, W.J.; Wright, A.L.; Guerra, S.; Martinez, F.D. Poor airway function in early infancy and lung function by age 22 years: A non-selective longitudinal cohort study. Lancet 2007, 370, 758–764. [Google Scholar] [CrossRef]
- Suresh, S.; O’Callaghan, M.; Sly, P.D.; Mamun, A.A. Impact of childhood anthropometry trends on adult lung function. Chest 2015, 147, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Lodge, C.J.; Allen, K.J.; Abramson, M.J.; Matheson, M.C.; Thomas, P.S.; Barton, C.; Bennett, C.; Erbas, B.; Svanes, C.; et al. Cohort Profile: Melbourne Atopy Cohort study (MACS). Int. J. Epidemiol. 2017, 46, 25–26. [Google Scholar] [CrossRef]
- Ali, G.B.; Bui, D.S.; Lodge, C.J.; Waidyatillake, N.T.; Perret, J.L.; Sun, C.; Walters, E.H.; Abramson, M.J.; Lowe, A.J.; Dharmage, S.C. Infant body mass index trajectories and asthma and lung function. J. Allergy Clin. Immunol. 2021, 148, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Baraghoshi, D.; Curran-Everett, D.; Zeiger, R.S.; Szefler, S.J.; Covar, R.A.; Williams, P.; Lasley, M.V.; Chinn, T.; Hinatsu, M.; et al. Factors Associated with Persistence of Severe Asthma from Late Adolescence to Early Adulthood. Am. J. Respir. Crit. Care Med. 2021, 204, 776–787. [Google Scholar] [CrossRef]
- Vogelberg, C.; Goldstein, S.; Graham, L.; Kaplan, A.; de la Hoz, A.; Hamelmann, E. A comparison of tiotropium, long-acting beta2-agonists and leukotriene receptor antagonists on lung function and exacerbations in paediatric patients with asthma. Respir. Res. 2020, 21, 19. [Google Scholar] [CrossRef]
- Chauhan, B.F.; Chartrand, C.; Ni, C.M.; Milan, S.J.; Ducharme, F.M. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst. Rev. 2015, 11, CD007949. [Google Scholar] [CrossRef]
- Kachroo, P.; Stewart, I.D.; Kelly, R.S.; Stav, M.; Mendez, K.; Dahlin, A.; Soeteman, D.I.; Chu, S.H.; Huang, M.; Cote, M.; et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 2022, 28, 814–822. [Google Scholar] [CrossRef]
| Background Factor | n#1 | Trajectory Type | ||||
|---|---|---|---|---|---|---|
| Normal n = 150 | Upward n = 47 | Downward n = 66 | Low n = 10 | p Value | ||
| Sex (boys), n (%) | 273 | 89 (59) | 30 (64) | 43 (65) | 6 (60) | 0.853 |
| Gestational age (weeks), median (range) | 210 | 39 (30–42) | 39 (28–41) | 39 (30–42) | 38 (24–41) | 0.698 |
| Birth weight (g), median (range) | 220 | 2968 (860–4494) | 3036 (1158–3896) | 3060 (1372–4050) | 3016 (845–3966) | 0.671 |
| Comorbid allergic diseases | ||||||
| Atopic dermatitis, n (%) | 272 | 79 (53) | 23 (49) | 28 (43) | 3 (30) | 0.367 |
| Perennial allergic rhinitis, n (%) | 272 | 92 (61) | 31 (66) | 31 (48) | 5 (50) | 0.172 |
| Seasonal allergic rhinitis, n (%) | 272 | 42 (28) | 14 (30) | 17 (26) | 2 (20) | 0.923 |
| Food allergy, n (%) | 270 | 78 (52) | 17 (37) | 32 (49) | 1 (10) | 0.027 |
| Other comorbidities | 273 | 13 (9) | 11 (23) | 7 (11) | 1 (10) | 0.045 |
| Family history and environment | ||||||
| Parental asthma, n (%) | 249 | 30 (22) | 8 (19) | 9 (15) | 3 (33) | 0.559 |
| Parental smoking, n (%) | 222 | 43 (34) | 14 (36) | 20 (40) | 4 (50) | 0.768 |
| Pet ownership, n (%) | 209 | 33 (27) | 7 (25) | 8 (16) | 3 (38) | 0.346 |
| Laboratory data #2 | ||||||
| Eosinophils (/μL), median (range) | 261 | 490 (0–3063) | 524 (0–1402) | 535 (100–2520) | 390 (118–1040) | 0.738 |
| Total-IgE (IU/mL), median (range) | 258 | 954 (10–12700) | 876 (39–7300) | 743 (4–10600) | 602 (99–8726) | 0.625 |
| HDM-sIgE (kUA/L), median (range) | 245 | 100 (<0.1–800) | 81.9 (<0.1–553) | 99.7 (<0.1–394) | 58.7 (0.5–436) | 0.729 |
| JCP-sIgE (kUA/L), median (range) | 248 | 31.5 (<0.1–468) | 22.9 (<0.1–553) | 34.7 (<0.1–477) | 28.7 (<0.1–249) | 0.715 |
| Asthma treatment before 6 years old | ||||||
| ICS | 252 | 75 (54) | 29 (64) | 37 (65) | 8 (80) | 0.179 |
| LTRA | 254 | 113 (80) | 45 (87) | 62 (76) | 10 (70) | 0.615 |
| Trajectory Type | ||||
|---|---|---|---|---|
| Normal | Upward | Downward | Low | |
| Z-Score for Height | ||||
| 6–9 years; mean | −0.066 | 0.232 | −0.221 | −0.121 |
| 95% CI | −0.219 to 0.0863 | −0.0617 to 0.527 | −0.469 to 0.0268 | −0.832 to 0.590 |
| n | 145 | 46 | 66 | 10 |
| p-value | n.s. | n.s. | n.s. | n.s. |
| 10–12 years; mean | −0.133 | 0.302 | −0.340 # | −0.133 |
| 95% CI | −0.283 to 0.0166 | −0.009 to 0.613 | −0.599 to −0.080 | −1.012 to 0.745 |
| n | 147 | 46 | 65 | 10 |
| p-value | n.s. | n.s. | 0.0112 | n.s. |
| 13–15 years; mean | −0.249 | 0.193 | −0.388 # | −0.181 |
| 95% CI | −0.399 to −0.0981 | −0.109 to 0.495 | −0.655 to −0.112 | −1.244 to 0.882 |
| n | 144 | 46 | 66 | 10 |
| p-value | 0.0014 | n.s. | 0.0052 | n.s. |
| Z-Score for BMI | ||||
| 6–9 years; mean | −0.271 | 0.079 | −0.330 | −0.692 |
| 95% CI | −0.422 to −0.119 | −0.248 to 0.406 | −0.562 to −0.099 | −1.453 to 0.070 |
| n | 145 | 46 | 66 | 10 |
| p-value | 0.0006 | n.s. | 0.0059 | n.s. |
| 10–12 years; mean | −0.296 | 0.011 | −0.357 | −0.655 |
| 95% CI | −0.448 to −0.144 | −0.304 to 0.326 | −0.567 to −0.147 | −1.366 to 0.056 |
| n | 147 | 46 | 65 | 10 |
| p-value | 0.0002 | n.s. | 0.0012 | n.s. |
| 13–15 years; mean | −0.205 | 0.242 | −0.280 | −0.851 |
| 95% CI | −0.383 to −0.028 | −0.117 to 0.602 | −0.518 to −0.0416 | −1.563 to −0.139 |
| n | 144 | 46 | 66 | 10 |
| p-value | 0.0238 | n.s. | 0.0221 | 0.0242 |
| Factor | Trajectory Type | p Value #1 | |
|---|---|---|---|
| Normal/Upward n = 197 | Downward/Low n = 76 | ||
| C-ACT or ACT≤ 19, n (%) | |||
| 6–9 years | 53 (28) | 21 (28) | n.s. |
| 10–12 years | 32 (17) | 26 (35) | 0.001 |
| 13–15 years | 14 (7) | 12 (16) | 0.030 |
| FeNO (ppb), median (range) | |||
| 6–9 years | 33 (1–142) | 37 (2–120) | n.s. |
| 10–12 years | 48 (7–197) | 53 (7–99) | n.s. |
| 13–15 years | 53 (7–165) | 47 (15–179) | n.s. |
| Treatment | |||
| ICS use | n (%) | ||
| 6–9 years | 154 (92) | 58 (92) | n.s. |
| 10–12 years | 161 (89) | 64 (90) | n.s. |
| 13–15 years | 109 (77) | 44 (80) | n.s. |
| High-dose ICS use#2 | n (%) | ||
| 6–9 years | 6 (4) | 4 (6) | n.s. |
| 10–12 years | 17 (9) | 4 (6) | n.s. |
| 13–15 years | 17 (12) | 3 (6) | n.s. |
| LABA use | n (%) | ||
| 6–9 years | 61 (36) | 32 (49) | n.s. |
| 10–12 years | 75 (40) | 44 (61) | 0.002 |
| 13–15 years | 51 (31) | 31 (55) | 0.001 |
| LTRA use | n (%) | ||
| 6–9 years | 138 (81) | 53 (79) | n.s. |
| 10–12 years | 167 (88) | 64 (89) | n.s. |
| 13–15 years | 93 (50) | 38 (57) | n.s. |
| Factor | OR | 95% CI | p-Value |
|---|---|---|---|
| Height Z-score in the 13–15 age period | 0.65 | 0.45–0.93 | 0.016 |
| ACT ≤ 19 in the 13–15 age period | 3.87 | 1.29–12.32 | 0.016 |
| ICS use in the 10–12 age period | 0.27 | 0.08–0.88 | 0.031 |
| High-dose ICS use in the 13–15 age period | 0.17 | 0.03–0.67 | 0.010 |
| LABA use in the 13–15 age period | 2.85 | 1.21–6.89 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Fujisawa, T.; Nagao, M.; Matsuzaki, H.; Motomura, C.; Odajima, H.; Nakamura, T.; Imai, T.; Nagakura, K.-i.; Yanagida, N.; et al. Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study. Children 2022, 9, 1516. https://doi.org/10.3390/children9101516
Yamada S, Fujisawa T, Nagao M, Matsuzaki H, Motomura C, Odajima H, Nakamura T, Imai T, Nagakura K-i, Yanagida N, et al. Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study. Children. 2022; 9(10):1516. https://doi.org/10.3390/children9101516
Chicago/Turabian StyleYamada, Shingo, Takao Fujisawa, Mizuho Nagao, Hiroshi Matsuzaki, Chikako Motomura, Hiroshi Odajima, Toshinori Nakamura, Takanori Imai, Ken-ichi Nagakura, Noriyuki Yanagida, and et al. 2022. "Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study" Children 9, no. 10: 1516. https://doi.org/10.3390/children9101516
APA StyleYamada, S., Fujisawa, T., Nagao, M., Matsuzaki, H., Motomura, C., Odajima, H., Nakamura, T., Imai, T., Nagakura, K.-i., Yanagida, N., Mitomori, M., Ebisawa, M., Kabashima, S., Ohya, Y., Habukawa, C., Tomiita, M., & Hirayama, M. (2022). Risk Factors for Lung Function Decline in Pediatric Asthma under Treatment: A Retrospective, Multicenter, Observational Study. Children, 9(10), 1516. https://doi.org/10.3390/children9101516