1. Introduction
A critical region of the lung, the small airways of pediatric asthmatics, can be a particularly difficult area to investigate and target with therapy. A recent review by Hopp et al. titled “
Small airways disease in pediatric asthma: the who, what, when, where, why, and how to remediate. A review and commentary” provides a wide-ranging discussion of the problem [
1]. The review covers the time course of the recognition of the problem, a comparison of diagnostic possibilities and a discussion of potential therapies [
1].
Recognized in the 1970′s as a “silent zone” of the lung, minimal attention is paid to any straightforward methodology of investigating its contribution to pediatric asthma [
1,
2,
3,
4]. Standard pulmonary function, however, provides a wealth of small airway data, which was outlined in reviews by Hopp et al. [
1,
5].
A 2014 review of techniques for assessing small airways dysfunction (less than 2 mm) reviewed information of spirometry, plethysmography, impulse oscillometry, single breath nitrogen washout, multiple breath nitrogen washout, helium and sulphur hexafluride washout, exhaled nitric oxide, high resolution computerized tomography (CT), hyperpolarized helium, magnetic resonance imaging (MRI), and nuclear medicine techniques of two-dimensional gamma scintigraphy, single photon emission computed tomography, and positron emission tomography [
4]. It is clear from the above list that only spirometry is readily available, with exhaled nitric oxide a distant second, especially in children.
A consortium study of adult asthmatics published in 2019 revealed that the single measure of Forced Vital Capacity (FVC) improvement after albuterol, followed by the forced mid-expiratory flow (FEF
25-75%) were both correlated to small airway dysfunction as defined by high-res CT [
6]. Lung clearance was not nearly as sensitive [
6].
A recent study in asthmatics found older, more obese, Type 2 inflammation and smoking to be related to both functional exercise capacity and diminished small airway measures of impedance oscillometry, FEF
25-75, and a lung clearance index [
7].
Another recent study in adult asthmatics showed monitoring FEF
25-75 has important outcome priorities [
8]. Of particular interest to our report, they designed a FEF
25-75 of less than 65% of predicted as their standard for stating a subject had airway dysfunction [
8].
Other recent studies of asthmatic children have also focused on SAD, using traditional and less readily available technology [
9,
10,
11].
To further focus the use of FEF25-75 in pediatric asthma outpatient medicine we present the results of a prospective analysis of small airway dysfunction in children initiated in April 2020 and completed in June 2021. All pulmonary function tests in pediatric asthmatics seen by a single asthma specialist (RJH) on those dates were included, with an analysis of differences in large and smaller airway function using only standardized spirometry.
The goal of the study was to determine if the presence of small airway dysfunction (SAD) was a priori and could be assigned as present (or not) based on the data available on the standard pulmonary function test. Small airway dysfunction was assigned based on a priori basis, with all elements of the PFT considered
prior to the group comparison analysis. The investigator providing the assignment of the J code for SAD (RJH) had recently published an extensive review of small airway disease in children [
1]. Bayesian probability is presumed to have properly sub-divided the total group into small and non-small airway dysfunction but the hypothesis of proper designation was tested with the post hoc statistical analysis.
4. Results
For the 16 months of the POC project 227 children were included in the analysis if they performed a satisfactory PFT as part of their asthma visit in the clinic of the author (RJH). A post hoc statistical analysis was made of the a priori small airway dysfunction code (J 98.4) done on the day of the visit.
One-hundred thirty-six children were assigned with a priori determined as not having small airway dysfunction, and 91 children were assigned to the small airway dysfunction code.
Table 1 presents the results of age, gender, baseline % predicted FVC, baseline FEV
1 percent predicted, FEF
25-75 percent predicted, z score for the FEF
25-75 and the numerical difference between the FEV
1 and the FEF
25-75 percent predicted. A gender difference was seen, as many of the a priori SAD males were a segment of the authors long-standing clinic practice. As this was a real-world study, albuterol was used
if a new patient or for medication step-up or step-down planning.
As seen in
Table 1, the a priori decision, using J code 98.4 strongly supported both a small airway and a large airway difference. FEV
1 precent predicted and the ratio of FEV1/FVC revealed large airway differences, while FEF
25-75 and z score for FEF
25-75 revealed smaller airway differences, both of a very significant nature. The unique use of product of the % predicted of FEV
1 (-) FEF
25-75 was to determine a proportional large airway and small airway numerical value, which was strongly statistically different between the two groups. The % predicted baseline FVC was not different between the groups, showing sustained volume, but obstructed lung function in the J98.4 group.
Using a cut-off of ≤65% for FEF25-75, none of the non-SAD (n = 136) had a value below the cut-off while 37 of the 91 SAD group did. The difference was significant at <0.00001. The mean for the SAD group for FEF25-75 was 66.9%.
For the possibility that a low FVC might negate the use of the FEF
25-75 [
15], the number of children below the −1.645 z score was determined. One child in the non-SAD group had an FVC z score of less than −1.645 while 3 of 91 children in the SAD group had a z score < −1.645, and the difference was not significant (
p > 0.05). However, removing those 4 subjects in a sub-group analysis did not change the significance of the data analysis.
Finally, a limited number of subjects also had a post-albuterol test, 41 (28%) of the non-SAD group and 38 (42%) of the SAD group. As this was a real-world study, albuterol was potentially used if it was a new patient or for potential medication step-up or step-down planning. For the post bronchodilator study, pre-post differences in FEV1 (≥9%), FEF25-75 (≥35%) and FVC (≥10%) were compared. The non-SAD vs. SAD group had no difference in FVC improvement, but a highly significant difference for improved FEV1 (p < 0.00001) and improved FEF25-75 (p < 0.00001) in the SAD group.
5. Discussion
The presence of small airway dysfunction is a current topic of investigation in the adult population and has been recently published in a predominately adult population using functional characteristics of physical activity and symptom control [
6,
7,
8]. Studies of this type can serve as a blueprint for prospective pediatric asthma investigation and potential earlier intervention, and are under-represented in the pediatric literature
A group of world-wide collaborators are actively small airway dysfunction (SAD) in adult asthma [
6]. A study is listed at ClinicalTrials.gov and sponsored by Chiesi Farmaceutici S.p.A., with a title of “
AssessmenT of smalL Airways involvemeNT In aSthma (ATLANTIS), is a multinational, multicenter, non-pharmacological intervention, cross-sectional and longitudinal study” [
6]. Their study goal is the discovery of a clinically relevant, easy to use methodology of determining SAD in adult asthmatics [
6]. Although much debate surrounds the assignment of SAD, most centers have ready availability of PFT testing, but less so other measures. The study of Postma supported FEF
25-75 as a reasonable surrogate for a CT of the chest [
6]. Their model was used for this protocol, only using readily available results (PFT) during COVID.
In this project, however, we first attempted to determine the burden of SAD in a pediatric asthmatic population in tertiary care setting population. The secondary goal was to statistically prove our a priori assignment of SAD and non-SAD children was correct. For the fifteen-month period, all the children and adolescents in our academic clinical practice were seen at one speciality hospital, due to COVID limitations. For this time-period impulse oscillometry was not available, nor body-box plethysmography. Both might be valuable in SAD measurements, but as mentioned FEF
25-75 and FVC are reasonable surrogates [
6,
8]. The sole physician assessing the patients, and presented in this study, used standardized pulmonary function results, obtained by hospital-based respiratory therapists. All subjects included were seen for routine asthma visits, or new asthma visits. The diagnosis of asthma had been previously determined (or was that day) and the known asthmatic subjects were on standard asthma therapy as defined by the NHLBI guidelines from 2007 or placed on that day based on severity [
16]. In the case of known asthma, pulmonary function tests utilizing albuterol were only done when medication step-up or down was being considered. New asthmatics were enrolled when the PFT pre-post albuterol demonstrated >a 12% change in FEV
1 along with the clinical decision the patient had asthma. Asthma medications were maintained or modified or started for each subject but are not delineated for this report. If nitric oxide was done, it was used as an independent variable to this report and was not included in the decision-making for the J 98.4 coding.
Once the children were assigned to SAD, the code J 98.4 was added as a separate asthma code in the electronic medical record. In part, the code was added for ease of analysis, but as seen in
Table 1 the assignment demonstrated very significant implications. Tests of total lung capacity and large airway function, FVC, FEV
1 and FEV1/FVC and z scores were analyzed.
The publication of Potsma et al. had suggested the FVC had a high predictability for a SAD phenotype (using chest CT as the gold standard) [
6]. Another study has shown a change in FVC > 10% had high clinical relevance as a marker of SAD [
17]. In this report there was not a significant difference in FVC between the two groups (
Table 1). Only 4 children in the entire study had an FVC z score less than −1.645. Among the subjects undergoing post-albuterol, only four subjects in each group improved their FVC > 10%. These data strongly represent preservation of FVC even in the face of marked obstruction in large (FEV
1) and smaller airways (FEF
25-75) in the small airway disease group.
In the large airway tests, FEV1 and FEV1/FVC ratios were significantly different between the groups. This significant difference shows both a large and smaller airway obstruction component for the SAD group. There was a significant improvement in FEV1 for twenty five of the 38 post-albuterol tests in the SAD group, and only eight of the 41 no-SAD group (p < 0.00001). The FEV1/FVC ratio difference reflected the concomitant large airway obstruction in the SAD group (p < 0.00001).
The FEF
25-75 was the value with the most clinical weight in distinguishing the two groups. The statistical analysis showed a highly significant difference (
p = 4.5 × 10
−35). Even without a post-albuterol test, the medication the subject was on at the time of the test was not sufficient, or in the case of a new patient indicated a higher step-care was needed. Equal to FEV
1 improvement was the improvement in FEF
25-75 in the thirty-eight who performed an albuterol challenge (25 improved) SAD group of >35% (
p < 0.00001). Only 8 of 41 non-SAD had improvement in FEF
25-75, but as a group they started at 108 ± 15%).
Figure 1 shows the box plot of the FEF
25-75. The boxplot suggests a FEF
25-75 percent predicted less than 80% will be a good cut-off for strongly suggesting SAD.
The unique analysis of the product of the % predicted of FEV
1-FEF
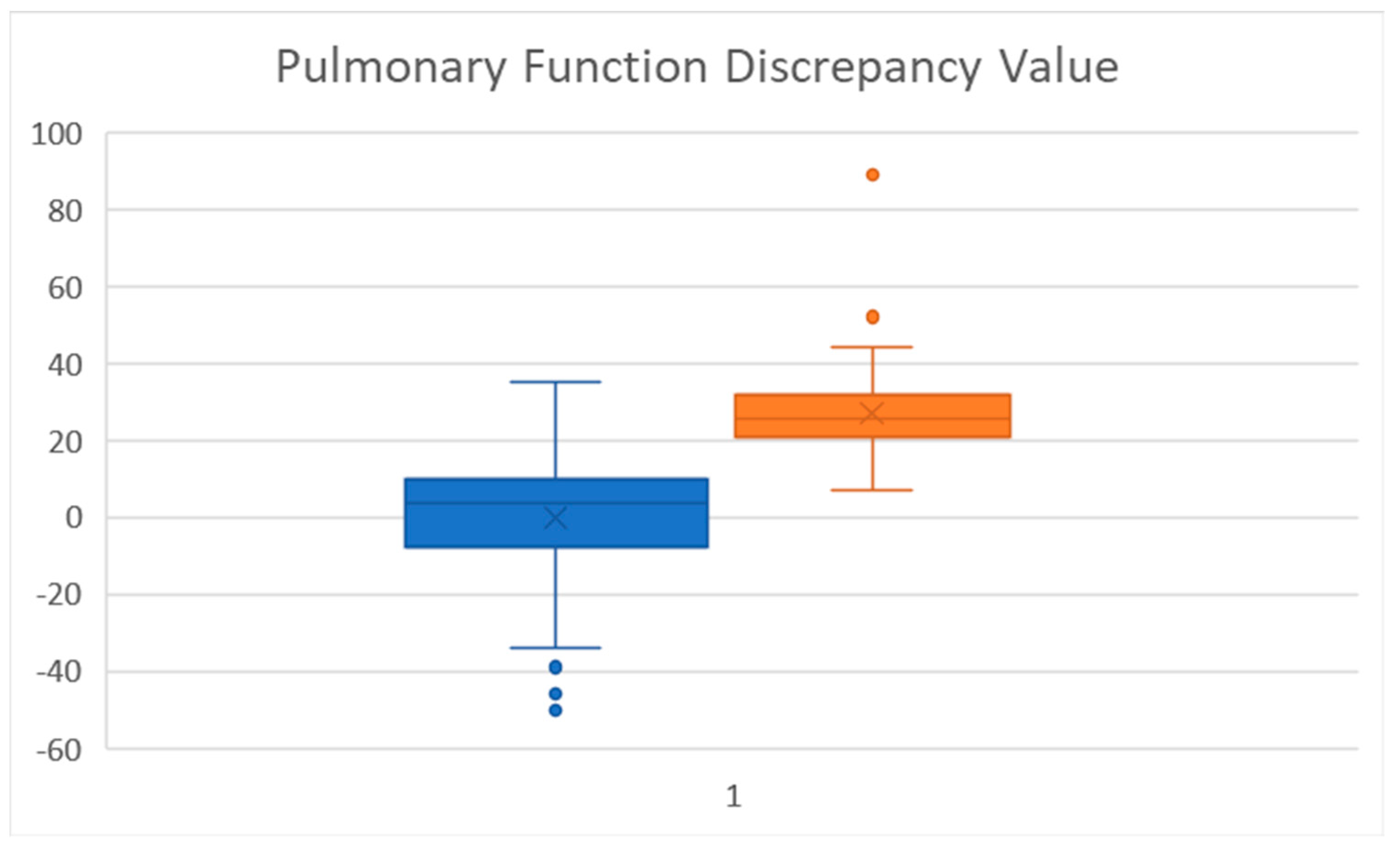
25-75 was also strongly significant (Pulmonary Function Discrepancy). To our knowledge, this determination has not been used previously used, but provides a simple number to benchmark. A boxplot of this value (
Figure 2) shows marked separation between the groups. A difference of 20 or more showed a large separation (at the 75 percentile) of the FEV
1% predicted (-) FEF
25-75% predicted. A larger prospective study would need to be done to further assess its applicability.
Small airway measures have had a less obvious position in pediatric asthma management [
1]. This is not as true in adult asthma, as recent efforts are underway to determine characteristics of small airway disease in adults [
6,
7,
8] and matched to their physiological findings [
8]. A recent report of mismatch of FEF
25-75 and FEV
1 in adult asthma as a measure of airway dysfunction has been published, which used FEF
25-75 as the marker of SAD [
8]. In that study, the FEF
25-75 was better than the FEV
1 in predicting airway hyperresponsiveness and severe asthma. They used a FEF
25-75 < 65% as marking small airway dysfunction. In our study, Thirty-nine of the 91 SAD children had a FEF
25-75 less than 65%, while 74% had a FEF
25-75 below 80%. So, in fact, the airways beyond the 7–8th generation (>2 mm) had less than an 80% capacity in the majority of the SAD group, with a mean FEF
25-75 for the SAD group of 66.9%.
Our report mirrors this data [
6,
7,
8], but in a pediatric population. In addition, a very recent study suggests impulse oscillometry (IOS) can be of further value in assessing large airway obstruction in adults [
18]. Their protocol only investigated FEV
1 and FVC baseline along with IOS testing. In our study, large airway measures plus more distal measures were compared. IOS was not included here, however, due to COVID restrictions during the study period.
SAD has been recently discussed in the pediatric asthma arena in two recent reviews [
1,
5]. Using information from the standard pulmonary function test we were able to show highly significant differences between the groups for smaller airway dynamics. FEF
25-75 and the z score for FEF
25-75 had markedly statistical differences (
Table 1) and had minimal overlap between the two groups. The unique continuous variable of the predicted FEV
1(-) FEF
25-75 was also significantly different with minimal over-lap between the 2 groups. It is possible this number bridges the boundary of a strictly large vs. small airway caliber dysfunction. It has not had previous usage and needs perspective analysis.
There are limitations to this study. The asthmatics were all of one investigator, and of a more significant nature as they were seen at a tertiary care hospital for children. The parents had to be able to arrive with their children/adolescents during the COVID-time period. The children self-selected, based on an in-person appointment, or post-telehealth visit for their PFT. In large part, it was a real-world protocol. Finally, there is no gold standard for absolutely establishing small airway disease, except for a CT, which was beyond the scope of this report. A recent adult-based protocol provided a model for this analysis [
6]. Other limitations include non-use of IOS or body-box plethysmography as supportive tests of SAD, but these tests require separate visits and were not allowed during the COVID period. Studies using IOS are useful in selected populations, but possibly less so when the base PFT answers most questions [
18].
The strengths include a single investigator providing the a priori classification, and consistent well-trained respiratory technicians doing the PFT studies in a hospital-based laboratory. The resultant data mirrors the evolving literature on SAD [
6,
7,
8,
18,
19]. This report provides a one-of-a-kind, real-world experience of determining the presence of smaller airway dysfunction using standard pulmonary function test analysis. IOS testing could add
further value [
18], but its use in our protocol was not included. In addition, suspecting SAD based on a pediatric PFT could allow for CT-scan or IOS support for SAD in a subsequent study, as is being done in young adults in an American-Lung Association-NHLBI sponsored prospective multi-centered study in young adults [
20], and the international study of adults [
6]. It may also serve as an indication for earlier step-up therapy, such as biologics.