Echocardiographic Screening for Rheumatic Heart Disease in a Ugandan Orphanage: Feasibility and Outcomes
Abstract
1. Introduction
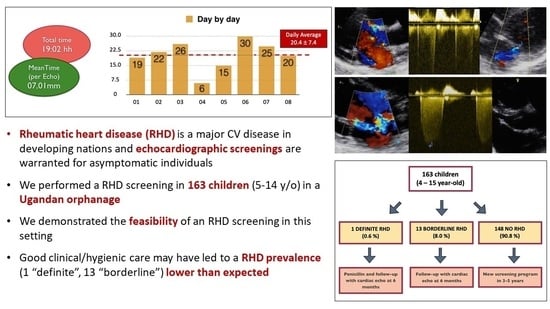
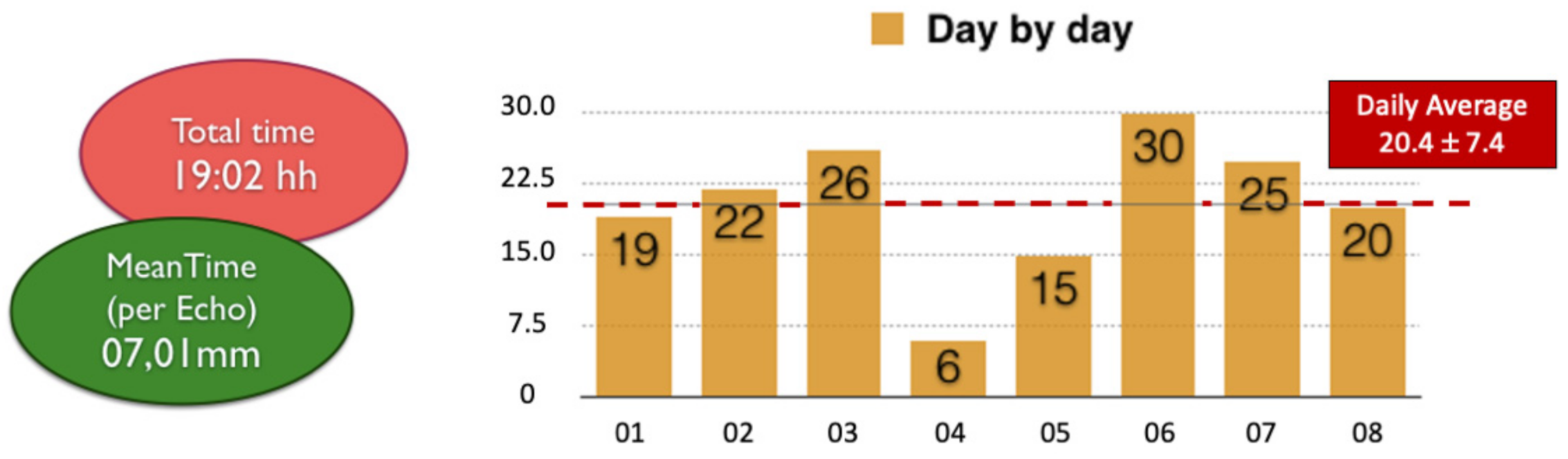
2. Materials and Methods
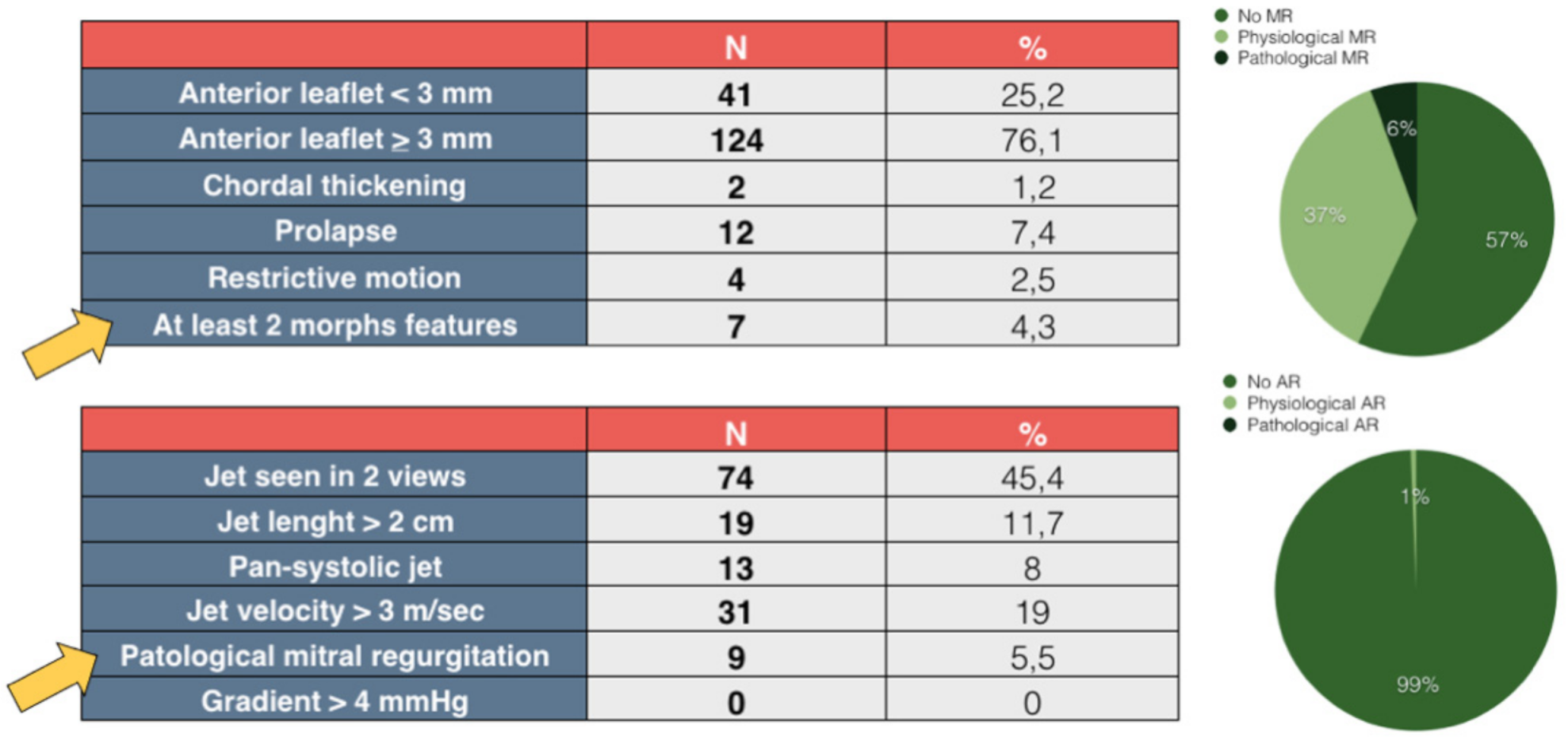
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Roberts, K.; Colquhoun, S.; Steer, A.; Reményi, B.; Carapetis, J. Screening for rheumatic heart disease: Current approaches and controversies. Nat. Rev. Cardiol. 2013, 10, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.; Longenecker, C.T.; Beaton, A.; Kamya, M.R.; Lwabi, P. Rheumatic heart disease in Uganda: Predictors of morbidity and mortality one year after presentation. BMC Cardiovasc. Disord. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Kayima, J.; Mungoma, M.; Mondo, C.; Freers, J. The Changing Pattern of Cardiac disease in Africa: The Ugandan Experience. Cardiovasc. J. Afr. 2011, 22, S9. [Google Scholar]
- Okello, E.; Zhang, W.; Musoke, C.; Aliku, T.; Kakande, B.; Mondo, C.K.; Freers, J.; Twalib, A.; Lwabi, P.; Wilson, N.B.; et al. Cardiovascular complications in newly diagnosed rheumatic heart disease patients at Mulago Hospital, Uganda. Cardiovasc. J. Afr. 2013, 24, 80–85. [Google Scholar] [CrossRef]
- Bland, E.; Jones, T. Rheumatic fever and rheumatic heart disease. A twenty-year report on 1000 patients followed since childhood. Circulation 1951, 4, 836–843. [Google Scholar] [CrossRef]
- Tompkins, D.G.; Boxerbaum, B.; Liebman, J. Long-term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation 1972, 1972, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Reményi, B.; Wilson, N.; Steer, A.; Ferreira, B.; Kado, J.; Kumar, K.; Lawrenson, J.; Maguire, G.; Marijon, E.; Mirabel, M.; et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat. Rev. Cardiol. 2012, 9, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Maguire, G.; Brown, A.; Atkinson, D.; Reményi, B.; Wheaton, G.; Kelly, A.; Kumar, R.K.; Su, J.-Y.; Carapetis, J. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation 2014, 129, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Zühlke, L.; Engel, M.E.; Karthikeyan, G.; Rangarajan, S.; Mackie, P.; Cupido, B.; Mauff, K.; Islam, S.; Joachim, A.; Daniels, R.; et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The Global Rheumatic Heart Disease Registry (the REMEDY study). Eur. Heart J. 2015, 36, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Meira, Z.M.; Goulart, E.M.; Colosimo, E.A.; Mota, C.C. Long term follow up of rheumatic fever and predictors of severe rheumatic valvar disease in Brazilian children and adolescents. Heart 2005, 91, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, S.; Khorsandi, M.; Herbst, P. Rheumatic heart disease screening: Current concepts and challenges. Ann. Pediatr. Cardiol. 2017, 10, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Celermajer, D.S.; Ferreira, B.; Tafflet, M.; Perier, M.C.; Karam, N.; Mocumbi, A.O.; Jani, D.N.; Sidi, D.; Jouven, X.; et al. Screening for rheumatic heart disease: Evaluation of a simplified echocardiography-based approach. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Beaton, A.; Lu, J.C.; Aliku, T.; Dean, P.; Gaur, L.; Weinberg, J.; Godown, J.; Lwabi, P.; Mirembe, G.; Okello, E.; et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: A field study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 475–482. [Google Scholar] [CrossRef]
- Lu, J.C.; Sable, C.; Ensing, G.J.; Webb, C.; Scheel, J.; Aliku, T.; Lwabi, P.; Godown, J.; Beaton, A. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J. Am. Soc. Echocardiogr. 2015, 28, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, S.M.; Carapetis, J.R.; Kado, J.H.; Reeves, B.M.; Remenyi, B.; May, W.; Wilson, N.J.; Steer, A.C. Pilot study of nurse-led rheumatic heart disease echocardiography screening in Fiji—A novel approach in a resource-poor setting. Cardiol. Young 2013, 23, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Bacquelin, R.; Tafflet, M.; Robillard, C.; Huon, B.; Corsenac, P.; de Frémicourt, I.; Narayanan, K.; Meunier, J.M.; Noël, B.; et al. Screening for rheumatic heart disease: Evaluation of a focused cardiac ultrasound approach. Circ. Cardiovasc. Imaging 2015, 8, e002324. [Google Scholar] [CrossRef] [PubMed]
- Ploutz, M.; Lu, J.C.; Scheel, J.; Webb, C.; Ensing, G.J.; Aliku, T.; Lwabi, P.; Sable, C.; Beaton, A. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart 2016, 102, 35–39. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapelli, M.; Zagni, P.; Calbi, V.; Fusini, L.; Twalib, A.; Ferrara, R.; Mattavelli, I.; Alberghina, L.; Salvioni, E.; Opira, C.; et al. Echocardiographic Screening for Rheumatic Heart Disease in a Ugandan Orphanage: Feasibility and Outcomes. Children 2022, 9, 1451. https://doi.org/10.3390/children9101451
Mapelli M, Zagni P, Calbi V, Fusini L, Twalib A, Ferrara R, Mattavelli I, Alberghina L, Salvioni E, Opira C, et al. Echocardiographic Screening for Rheumatic Heart Disease in a Ugandan Orphanage: Feasibility and Outcomes. Children. 2022; 9(10):1451. https://doi.org/10.3390/children9101451
Chicago/Turabian StyleMapelli, Massimo, Paola Zagni, Valeria Calbi, Laura Fusini, Aliku Twalib, Roberto Ferrara, Irene Mattavelli, Laura Alberghina, Elisabetta Salvioni, Cyprian Opira, and et al. 2022. "Echocardiographic Screening for Rheumatic Heart Disease in a Ugandan Orphanage: Feasibility and Outcomes" Children 9, no. 10: 1451. https://doi.org/10.3390/children9101451
APA StyleMapelli, M., Zagni, P., Calbi, V., Fusini, L., Twalib, A., Ferrara, R., Mattavelli, I., Alberghina, L., Salvioni, E., Opira, C., Kansiime, J., Tamborini, G., Pepi, M., & Agostoni, P. (2022). Echocardiographic Screening for Rheumatic Heart Disease in a Ugandan Orphanage: Feasibility and Outcomes. Children, 9(10), 1451. https://doi.org/10.3390/children9101451