Successful Pulmonary Endarterectomy after Acute Pulmonary Embolism and Reversal of Acute Cor Pulmonale in an 11-Year-Old Boy with Nephrotic Syndrome
Abstract
:1. Introduction
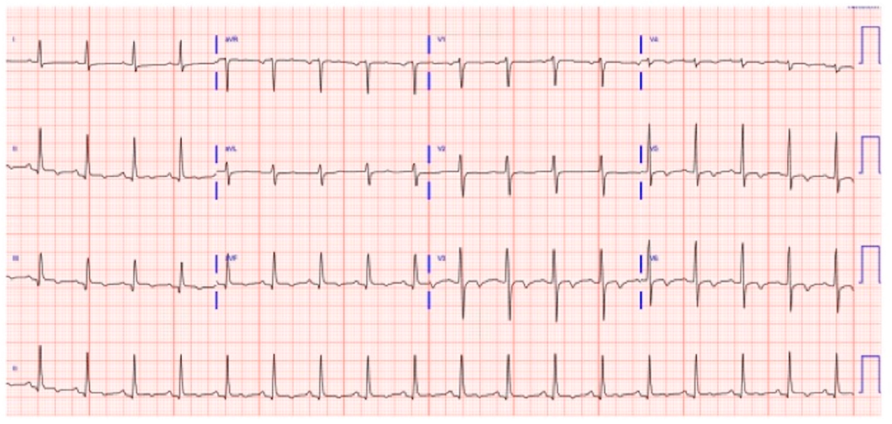
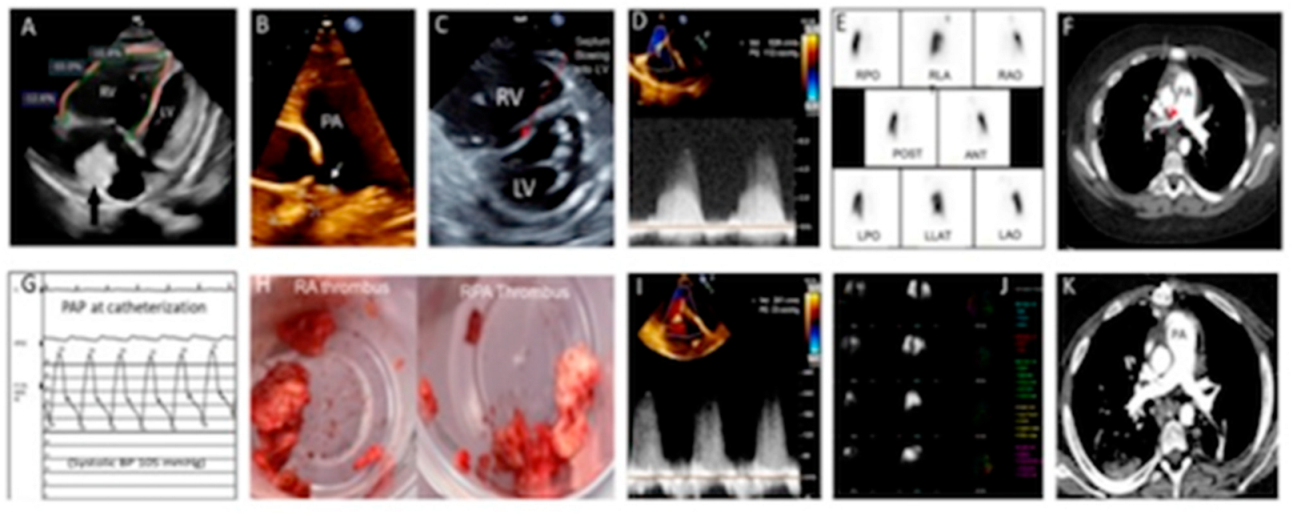
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller, A.P.; Troost, J.P.; Parikh, S.V.; Wolfgang, K.J.; Rovin, B.H.; Nieman, M.T.; Smoyer, W.E.; Kretzler, M.; Kerlin, B.A.; Neptune Investigators. Nephrotic syndrome disease activity is proportional to its associated hypercoagulopathy. Thromb. Res. 2021, 201, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Safdar, O.Y.; Rajab, R.H.; Alghanemi, R.G.; Tantawi, G.A.; Alsulami, N.A.; Alsayed, A.A.; Habiballah, A.K. Bilateral Pulmonary Embolism in a 12-Year-Old Girl with Steroid-Resistant Nephrotic Syndrome. Children 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qi, J.; Schoepf, J.; Savage, R.H.; Tang, C.; Lu, M.; Zhou, C.; Lu, G.; Wang, D.; Zhang, L. Prevalence and Associated Risk Factors of Pulmonary Embolism in Children and Young Adults with Nephrotic Syndrome: A Chinese Large Cohort Study. J. Thorac. Imaging 2021, 36, 326–332. [Google Scholar] [CrossRef]
- Kerlin, B.A.; Ayoob, R.; Smoyer, W.E. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Biss, T.T.; Brandao, L.R.; Kahr, W.H.; Chan, A.K.; Williams, S. Clinical probability score and D-dimer estimation lack utility in the diagnosis of childhood pulmonary embolism. J. Thromb. Haemost. 2009, 7, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.J.; Huang, S.; Uberoi, R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst. Rev. 2022, 8, CD013083. [Google Scholar]
- Ji, D.; Gill, A.E.; Durrence, W.; Shah, J.H.; Paden, M.L.; Patel, K.N.; Williamson, J.L.; Hawkins, C.M. Catheter-Directed Pharmacologic Thrombolysis for Acute Submassive and Massive Pulmonary Emboli in Children and Adolescents-An Exploratory Report. Pediatr. Crit. Care Med. 2020, 21, e15–e22. [Google Scholar] [CrossRef]
- Male, C.; Lensing, A.W.A.; Palumbo, J.S.; Kumar, R.; Nurmeev, I.; Hege, K.; Bonnet, D.; Connor, P.; Hooimeijer, H.L.; Torres, M.; et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: A randomized, controlled, phase 3 trial. Lancet Haematol. 2019, 7, e18–e27. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Michelson, A.D.; Van Bergen, A.H.; Horowitz, E.S.; Sandoval, J.P.; Justino, H.; Harris, K.C.; Jefferies, J.L.; Pina, L.M.; Peluso, C.; et al. Thromboprophylaxis for children post-Fontan procedure: Insights from the UNIVERSE study. J. Am. Hear. Assoc. 2021, 10, e021765. [Google Scholar] [CrossRef]
- Sanchez, O.; Trinquart, L.; Colombet, I.; Durieux, P.; Huisman, M.V.; Chatellier, G.; Meyer, G. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: A systematic review. Eur. Heart J. 2008, 29, 1569–1577. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American society of hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef] [PubMed]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef]
- Kumbasar, U.; Aypar, E.; Karagoz, T.; Demircin, M.; Dogan, R. Pulmonary thromboendarterectomy in pediatric patients: Report of three cases. Turk. J. Pediatr. 2018, 60, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Durand, P.; Devictor, D.; Planche, C.; Serraf, A. Unilateral right pulmonary thromboendarterectomy for chronic embolism: A successful procedure in an infant. J. Thorac. Cardiovasc. Surg. 1999, 118, 953–954; discussion 957. [Google Scholar] [CrossRef]
- Caeymaex, L.; Durand, P.; Lambert, V.; Retbi, J.M.; Devictor, D. Chronic cor pulmonale and refractory hypoxemia following pulmonary embolism in a six-month-old infant: Surgical management by thromboendarterectomy. Arch. Pediatr. 2000, 7, 851–854. [Google Scholar] [CrossRef]
- Zacharias, J.; Clark, S.C.; Hamilton, J.R.; Dark, J.H.; Hasan, A. Unilateral pulmonary thromboendarterectomy for iatrogenic pulmonary hypertension in a ten-year-old child. J. Thorac. Cardiovasc. Surg. 2003, 126, 1210–1211. [Google Scholar] [CrossRef]
- Verbelen, T.; Cools, B.; Fejzic, Z.; Van Den Eynde, R.; Maleux, G.; Delcroix, M.; Meyns, B. Pulmonary endarterectomy in a 12-year-old boy with multiple comorbidities. Pulm. Circ. 2019, 9, 2045894019886249. [Google Scholar] [CrossRef]
- Wang, A.S.; Rosenzweig, E.B.; Takeda, K. A rare childhood case of Behcet’s disease and chronic thromboembolic pulmonary hypertension. J. Card. Surg. 2020, 35, 1669–1672. [Google Scholar] [CrossRef]
- Madani, M.M.; Wittine, L.M.; Auger, W.R.; Fedullo, P.F.; Kerr, K.M.; Kim, N.H.; Test, V.J.; Kriett, J.M.; Jamieson, S.W. Chronic thromboembolic pulmonary hypertension in pediatric patients. J. Thorac. Cardiovasc. Surg. 2011, 141, 624–630. [Google Scholar] [CrossRef]
- Mkalaluh, S.; Szczechowicz, M.; Karck, M.; Szabo, G. Twenty-year results of surgical pulmonary thromboembolectomy in acute pulmonary embolism. Scand. Cardiovasc. J. 2019, 53, 98–103. [Google Scholar] [CrossRef]
- Bozenkova, E.; Soucek, M. Rivaroxaban for the treatment and prevention of recurrence of venous thromboembolism in children. Vnitr. Lek. 2021, 67, 165–168. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osakwe, O.; B. Das, B. Successful Pulmonary Endarterectomy after Acute Pulmonary Embolism and Reversal of Acute Cor Pulmonale in an 11-Year-Old Boy with Nephrotic Syndrome. Children 2022, 9, 1444. https://doi.org/10.3390/children9101444
Osakwe O, B. Das B. Successful Pulmonary Endarterectomy after Acute Pulmonary Embolism and Reversal of Acute Cor Pulmonale in an 11-Year-Old Boy with Nephrotic Syndrome. Children. 2022; 9(10):1444. https://doi.org/10.3390/children9101444
Chicago/Turabian StyleOsakwe, Onyekachukwue, and Bibhuti B. Das. 2022. "Successful Pulmonary Endarterectomy after Acute Pulmonary Embolism and Reversal of Acute Cor Pulmonale in an 11-Year-Old Boy with Nephrotic Syndrome" Children 9, no. 10: 1444. https://doi.org/10.3390/children9101444
APA StyleOsakwe, O., & B. Das, B. (2022). Successful Pulmonary Endarterectomy after Acute Pulmonary Embolism and Reversal of Acute Cor Pulmonale in an 11-Year-Old Boy with Nephrotic Syndrome. Children, 9(10), 1444. https://doi.org/10.3390/children9101444






