Pediatric Urachal Anomalies: Monocentric Experience and Mini-Review of Literature
Abstract
:1. Introduction
2. Methodology
2.1. Patients
2.2. Study Design
2.3. Surgery
2.4. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Age-Dependent Characteristics
3.3. Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, H.; Donohoe, J. Diagnosis and management of urachal anomalies in children. Curr. Bladder Dysfunct. Rep. 2015, 10, 256–263. [Google Scholar] [CrossRef]
- Sato, H.; Furuta, S.; Tsuji, S.; Kawase, H.; Kitagawa, H. The current strategy for urachal remnants. Pediatr. Surg. Int. 2015, 31, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.M.; Bowlin, P.R.; Bagli, D.J.; Lorenzo, A.J.; Hassouna, T.; Koyle, M.A.; Farhat, W.A. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J. Urol. 2015, 193, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Naiditch, J.A.; Radhakrishnan, J.; Chin, A.C. Current diagnosis and management of urachal remnants. J. Pediatr. Surg. 2013, 48, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Hashimoto, H.; Yokoyama, H.; Ito, M.; Kouda, K.; Kanamaru, H. Urachal anomalies: Ultrasonography and management. J. Pediatr. Surg. 2003, 38, 1203–1207. [Google Scholar] [CrossRef]
- Galati, V.; Donovan, B.; Ramji, F.; Campbell, J.; Kropp, B.P.; Frimberger, D. Management of urachal remnants in early childhood. J. Urol. 2008, 180, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. Int. J. Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.J.; Lemeshow, S.; Sturdivant, R. Applied Logistic Regression, 3rd ed.; Wiley: Hoboken, NJ, USA, 2013; Available online: http://lib.ugent.be/catalog/rug01:002052663 (accessed on 3 October 2021).
- Robert, Y.; Hennequin-Delerue, C.; Chaillet, D.; Dubrulle, F.; Biserte, J.; Lemaitre, L. Urachal remnants: Sonographic assessment. J. Clin. Ultrasound 1996, 24, 339–344. [Google Scholar] [CrossRef]
- Lipskar, A.M.; Glick, R.D.; Rosen, N.G.; Layliev, J.; Hong, A.R.; Dolgin, S.E.; Soffer, S.Z. Nonoperative management of symptomatic urachal anomalies. J. Pediatr. Surg. 2010, 45, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Stopak, J.K.; Azarow, K.S.; Abdessalam, S.F.; Raynor, S.C.; Perry, D.A.; Cusick, R.A. Trends in surgical management of urachal anomalies. J. Pediatr. Surg. 2015, 50, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Badawi, N.; Holland, A.J.A.; Halliday, R. Developmental outcomes following major surgery: What does the literature say? J. Paediatr. Child Health 2011, 47, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Halliday, R.; Holland, A.J.A.; Karskens, C.; Badawi, N. Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. J. Pediatr. Surg. 2010, 45, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- DiMaggio, C.; Sun, L.S.; Kakavouli, A.; Byrne, M.W.; Li, G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J. Neurosurg. Anesthesiol. 2009, 21, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellon, R.D.; Simone, A.F.; Rappaport, B.A. Use of anesthetic agents in neonates and young children. Anesth. Analg. 2007, 104, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.A.; Inman, B.A.; Routh, J.C.; Rohlinger, A.L.; Husmann, D.A.; Kramer, S.A. Urachal anomalies: A longitudinal study of urachal remnants in children and adults. J. Urol. 2007, 178, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- McCollum, M.O.; MacNeily, A.E.; Blair, G.K. Surgical implications of urachal remnants: Presentation and management. J. Pediatr. Surg. 2003, 38, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Cilento, B.G.; Bauer, S.B.; Retik, A.B.; Peters, C.; Atala, A. Urachal anomalies: Defining the best diagnostic modality. Urology 1998, 52, 120–122. [Google Scholar] [CrossRef]
- Minevich, E.; Wacksman, J.; Lewis, A.G.; Bukowski, T.P.; Sheldon, C.A. The infected urachal cyst: Primary excision versus a staged approach. J. Urol. 1997, 157, 1869–1872. [Google Scholar] [CrossRef]
- Hoffmann, F.; Bachmann, C.J. Differences in sociodemographic characteristics, health, and health service use of children and adolescents according to their health insurance funds. Bundesgesundheitsblatt Gesundh. Gesundh. 2014, 57, 455–463. [Google Scholar] [CrossRef]
| Σ | Age < 1 Year | Age > 1 Year | p | |
|---|---|---|---|---|
| n, (%) | 52 | 35 (67) | 17 (33) | |
| Age (months) | 3 (2–33) | 2 (1- 3) | 134 (33–148) | <0.001 ‡ |
| Body weight (kg) | 13.4 ± 16.8 | 5.2 ± 1.3 | 34.0 ± 20.0 | <0.001 † |
| Body length (cm) | 84 ± 41 | 57 ± 6 | 134 ± 32 | <0.001 † |
| Body mass index (kg/m2) | 16.3 ± 2.8 | 15.5 ± 1.9 | 17.9 ± 3.5 | 0.037 † |
| Gender (f:m) | 24:28 | 18:17 | 6:11 | 0.38 * |
| Body temperature on admission (°C) | 37.0 ± 0.5 | 37.0 ± 0.4 | 37.1 ± 0.7 | 0.78 † |
| Health insurance state n, (%) | ||||
| private/statutory | 5/47 | 3/32 (9) | 2/15 (12) | >0.99 * |
| Symptoms on admission n, (%) | ||||
| -umbilical discharge | 44 (85) | 31 (89) | 13 (77) | 0.41 * |
| -abdominal pain | 9 (17) | 0 | 9 (53) | 0.001 * |
| -abdominal mass | 2 (4) | 0 | 2 (12) | 0.10 * |
| -erythema | 31 (60) | 20 (57) | 11 (42) | 0.77 * |
| -fever | 2 (4) | 0 | 2 (12) | 0.10 * |
| -dysuria | 1 (2) | 0 | 1 (6) | 0.33 * |
| Procedural | ||||
| -post-surgical hospital stay | 3 (2–4) | 2 (2–4) | 3 (2–4) | 0.91 ‡ |
| -surgery duration | 63 ± 38 | 58 ± 36 | 72 ± 42 | 0.24 † |
| Overall complications n, (%) | ||||
| Σ | 10 (20) | 4 (12) | 6 (36) | 0.062 * |
| -pre-surgical onset n, (%) | 5 (10) | 0 | 5 (30) | 0.003 * |
| peritonitis | 3 (6) | 0 | 3 (18) | 0.031 * |
| pre-fascial abscess | 2 (4) | 0 | 2 (12) | 0.10 * |
| -post-surgical onset n, (%) | 5 (10) | 4 (12) | 1 (6) | 0.66 * |
| peritonitis | 1 (2) | 1 (3) | 0 | >0.99 * |
| pre-fascial abscess | 4 (8) | 3 (9) | 1 (6) | >0.99 * |
| Recurrence management n, (%) | ||||
| Σ | 5 (10) | 4 (12) | 1 (6) | 0.66 * |
| -conservative | 2 (4) | 2 (6) | 0 | 0.55 * |
| -surgical | 3 (6) | 2 (6) | 1 (6) | >0.99 * |
| Complicated | Non-Complicated | p | |
|---|---|---|---|
| n, (%) | 10 (19) | 42 (81) | |
| Age (months) | 42 (2–147) | 2 (1–17) | 0.018 ‡ |
| Body weight (kg) | 14.9 (6.0–39.5) | 5.7 (4.5–8.2) | 0.013 ‡ |
| Body length (cm) | 122 (61–158) | 61 (54–90) | 0.07 ‡ |
| Body mass index (kg/m2) | 16.9 ± 0.8 | 16.2 ± 3.1 | 0.24 † |
| Gender (f:m) | 6:4 | 18:24 | 0.48 * |
| Body temperature on admission (°C) | 37.2 ± 0.8 | 37.0 ± 0.5 | 0.60 † |
| Symptoms on admission n, (%) | |||
| -umbilical discharge | 7 (70) | 37 (88) | 0.17 * |
| -abdominal pain | 5 (50) | 4 (10) | 0.008 * |
| -abdominal mass | 2 (20) | 0 | 0.034 * |
| -erythema | 6 (60) | 23 (55) | >0.99 * |
| -dysuria | 1 (10) | 0 | 0.19 * |
| Laboratory on admission | |||
| CRP (mg/dL) | 5.8 ± 9.7 | 0.4 ± 0.8 | 0.20 † |
| WBC (x109/L) | 16.7 ± 3.5 | 10.8 ± 3.7 | <0.001 † |
| Platelets (x109/L) | 483 ± 151 | 459 ± 209 | 0.79 † |
| Hematocrit (%) | 36 ± 5 | 37 ± 13 | 0.77 † |
| Hemoglobin (g/dL) | 12.3 ± 1.6 | 13.2 ± 2.9 | 0.45 † |
| Procedural | |||
| Post-surgical hospital stay | 4 (3–9) | 2 (2–4) | 0.016 ‡ |
| Surgery duration | 73 ± 50 | 60 ± 35 | 0.34 † |
| Diagnostic mode n, (%) | |||
| Ultrasound | 6 (60) | 25 (60) | >0.99 * |
| Surgery | 4 (40) | 17 (40) | >0.99 * |
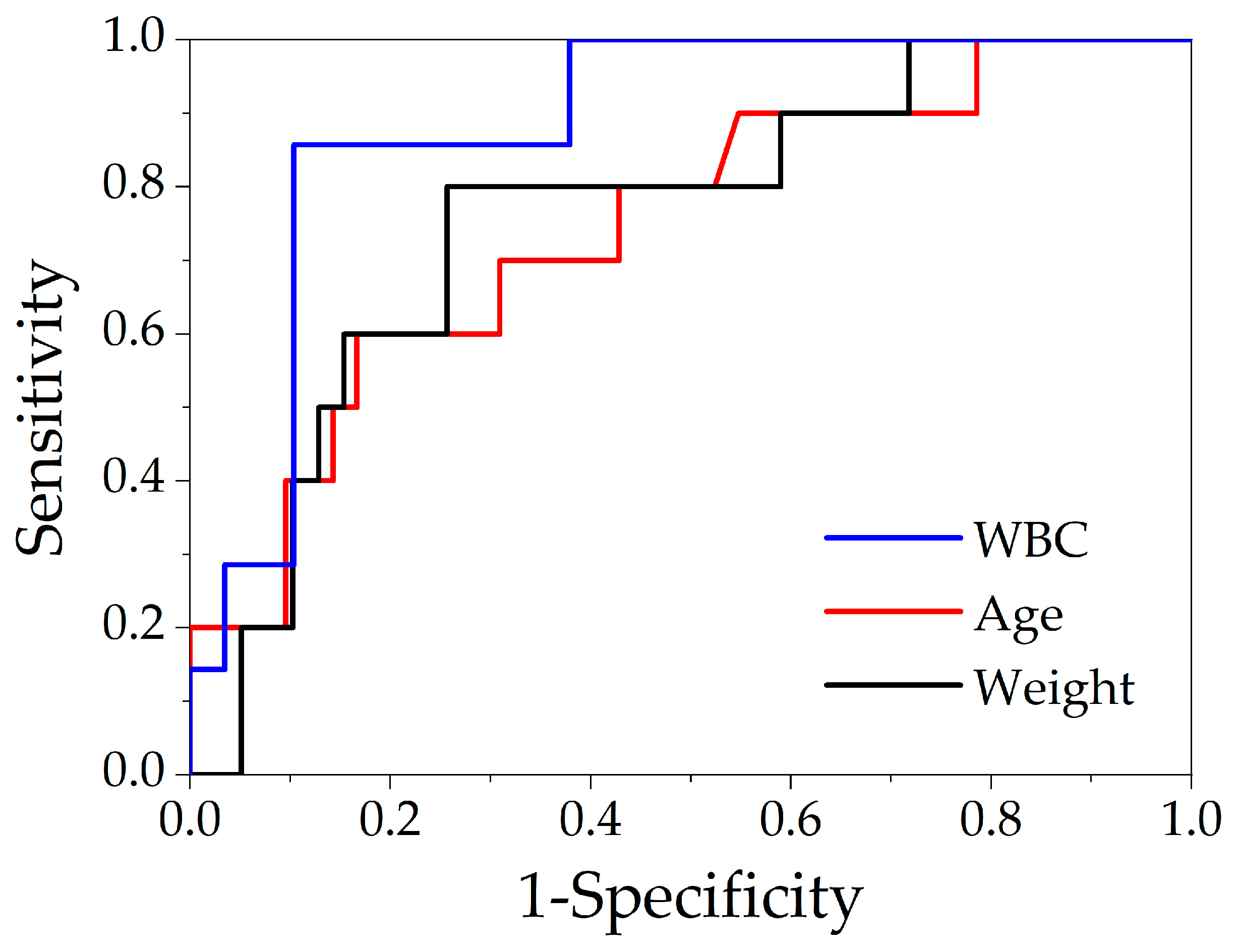
| AUC (±SE) | Cut-Off | Sensitivity | Specificity | 95% CI | p | OR (95%CI) | |
|---|---|---|---|---|---|---|---|
| WBC | 0.88 ± 0.09 | ≥14.3 × 109/L | 86% | 90% | 0.71–1.06 | 0.002 | 52 (4.57–591.30) |
| Age | 0.74 ± 0.09 | ≥5 months | 70% | 69% | 0.58–0.91 | 0.017 | 5.2 1(1.16–23.39) |
| Weight | 0.76 ± 0.09 | ≥6.2 kg | 80% | 74% | 0.58–0.94 | 0.012 | 11.6 (2.10–64.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, M.; Rogge, P.; Sander, V.; Alrefai, M.; Romanova, A.; Tröbs, R.-B. Pediatric Urachal Anomalies: Monocentric Experience and Mini-Review of Literature. Children 2022, 9, 72. https://doi.org/10.3390/children9010072
Nissen M, Rogge P, Sander V, Alrefai M, Romanova A, Tröbs R-B. Pediatric Urachal Anomalies: Monocentric Experience and Mini-Review of Literature. Children. 2022; 9(1):72. https://doi.org/10.3390/children9010072
Chicago/Turabian StyleNissen, Matthias, Phillip Rogge, Volker Sander, Mohamad Alrefai, Anna Romanova, and Ralf-Bodo Tröbs. 2022. "Pediatric Urachal Anomalies: Monocentric Experience and Mini-Review of Literature" Children 9, no. 1: 72. https://doi.org/10.3390/children9010072
APA StyleNissen, M., Rogge, P., Sander, V., Alrefai, M., Romanova, A., & Tröbs, R.-B. (2022). Pediatric Urachal Anomalies: Monocentric Experience and Mini-Review of Literature. Children, 9(1), 72. https://doi.org/10.3390/children9010072






