The Importance of Rhythmic Stimulation for Preterm Infants in the NICU
Abstract
:1. Introduction
1.1. Rhythm Perception In Utero
1.1.1. Maternal Heartbeat
1.1.2. Maternal Breathing
1.1.3. The Mother’s Voice
1.1.4. Maternal Walk
1.1.5. Intersensory Redundancy
1.1.6. Links between Multimodal Fetal Rhythms and Music
1.2. Rhythm Production In Utero
1.3. Rhythmic Stimulation in NICU
1.3.1. Rhythmic Vestibular Stimulation
1.3.2. Rhythmic Breathing Stimulation
1.3.3. Rhythmic Sucking Stimulation
1.3.4. Rhythmic Multimodal Stimulation
- Vestibular and heartbeat sound: Vestibular rhythmic stimulation shows different effects, depending on the stimuli, the rhythms chosen, the frequency of the stimuli, the quantity of stimuli proposed, and the duration of intervention. Always seeking to be as close as possible to the stimulations provided in utero, many authors have proposed multi-modal rhythmic stimulations by combining, for example, rocking with intrauterine cardiac noise and female voice [74] or a rocker bed with a heartbeat sound, whether or not it is triggered by the preterm infant [75]. The rhythmical stimulation could take place for 15 min/h (fixed-interval stimulation group); the infant provoked 15 min of rhythmic stimulation each time it was motorically inactive for 90 s (self-activating stimulation group), or the infant provoked 15 min of rhythmic stimulation by being inactive for 90 s, but the stimulation took place only once per hour for the fixed-interval stimulation group (quasi self-activating stimulation group). As in the original study [76], compared with the control group (without any rhythmical stimulation), the immediate effect of the rhythmical stimulation was an increase in quietness in the three experimental groups, with fewer abnormal reflexes and better orienting responses. The authors highlighted the importance of contingency (that the infant can produce an action, such as triggering the rhythmic movement of the bed) and the temporal pattern of stimulation (that it occurs once every hour).
- Multisensory stimulations, including rhythmical stimulation: Interventions of two programs incorporate rhythmical stimulations in a multisensory stimulation program.
- Mother’s voice and heartbeat sound: Studies have stimulated preterm infants with the mother’s voice and heartbeat at 30 min intervals, four times per 24 h [89]. Rhythmical maternal sound stimulation (MSS) starts within 7 days after birth and is continued until discharge from the NICU. Maternal sound and heartbeat were recorded for each infant during maternal speaking, reading, and singing. The authors found an overall decreasing trend in cardiorespiratory events (CREs) with age. With nearly the same stimulation (audio-recording of the mother’s voice and heartbeat sounds, four times per day for a duration of 45 min each over a period of 1 month), Webb et al. [90] used cranial ultrasonography measurements at 30 days of life. Extremely preterm infants exposed to their mothers’ voices and heartbeats during their first month of life in an incubator had significantly larger auditory cortexes bilaterally compared with control preterm infants, although the mother’s actual voice and live heartbeat would be even more effective [91].
- Music: Music interventions are especially difficult to fully describe due to the complexity of music stimuli (rhythm, pitch, tempo, harmonic, structure, timbre, jitter, shimmer, etc.), variety of music experiences, and factors due to music interventions. It is therefore difficult to know the effects of the rhythm itself. We only know that there is no music without rhythm, whereas there can be music without melody [42]. Music has often been effectively used in neonatal intensive care units, especially with high-risk infants [14]. Moreover, music is thought to improve neurodevelopment in preterm infants by promoting synaptic plasticity and the differentiation, activation, readjustment, and growth of neurons [92]. A review of music therapy in the NICU between 1970 and 2010 revealed previously unsuspected perceptual, adaptative, and active engagement capacities of preterm infants during music therapy [93]. The authors focused on music or auditory stimulation interventions that incorporated musical elements, such as rhythm and sounds, based on the acoustic rhythmic intrauterine environment, such as recorded womb sounds, the mother’s voice, breathing sounds, and heartbeats. The review showed that music has positive effects on the preterm infant, calming and relaxing the infant and decreasing its stress level. Another systematic review of music-based intervention research published from 2010 to 2015 showed poor quality of music intervention studies [94]. The authors recommended improving the reporting quality, scientific rigor, and clinical relevance of music intervention research and suggested a seven-component checklist to advance the scientific rigor and clinical relevance of music intervention research. A recent study showed that preterm infants can learn and memorize from their auditory environment and that they can discriminate music played in the neonatal unit from the same music with a faster tempo [18]. Preterm infants are therefore able to recognize the temporal structure of a known piece of music at a specific tempo and to differentiate it from the same piece played at a faster tempo. Rhythm processing has further been shown to be especially important for language processing and recognition. Early postnatal music intervention increases neural responses related to music tempo processing and recognition [95].
- Voice: Similar to the fetus and the full-term newborn, the preterm infant reacts more to its mother’s voice by displaying accelerated cardiac rhythm compared to when the voice is absent [96]. Just as the contingent voice is important to the infant’s responses [91], better self-regulation of the preterm infant has been observed during the interaction when the song is contingent to the infant’s reactions [97]. Similarly, the beneficial effects of singing are greater when the parents sing directly to the child versus when the mother sings as if her child were present [98]. Linguistic research shows that lullabies of all cultures combine language information and use calming, rhythmic stimuli. Lullabies, with no tempo change, were used to reinforce non-nutritive sucking rates of preterm infants. Contingent lullabies, such as pacifier-activated lullabies (PALs), increase pacifier-sucking rates of preterm infants [99], increase subsequent feeding rates [100], and shorten gavage feeding lengths when used at the specific gestation age of 34 weeks [101]. Rhythmic lullabies reinforce the sucking rates produced by preterm infants. Consequently, sucking rhythms are modified by lullabies: the more the preterm infant sucks, the more the lullabies provided. The preterm infant can learn to suck–swallow–breathe with music contingency.
- Kangaroo care: The importance of the multiplicity of rhythms in synchrony with each other and their possible link with musical rhythms has been shown in utero [10]. In the neonatal unit, the different rhythms are clearly less numerous and are only rarely presented together. The rare moment when the infant is again simultaneously in the presence of several rhythms in synchrony is when it is exposed to kangaroo care on the mother’s chest (skin-to-skin contact). The full-body contact and the sound of the mother’s heartbeat are thought to simulate sensations that the infant experienced prenatally [102]. In this position, the infant can again hear its mother’s heartbeat, perceive her breathing rhythm, and hear the rhythm of her speech if she is speaking or the rhythm of the song if she is singing. Intersensory redundancy is again present in a skin-to-skin-contact situation. When a mother speaks to her child (infant-directed speech), she uses the motherese, which accentuates the melodic contours and uses a slower rhythm, better perceived by the child [103]. Similarly, the infant-directed singing, used by the mother when she sings to her child, has more accentuated melodic contours and a slower rhythm that is better perceived by the child [104]. Parent–infant skin-to-skin contact, commonly known as kangaroo care, underscores the importance of maternal body contact for the infant’s physiological, emotional, and cognitive regulatory capacities [105]. Compared with kangaroo care alone, combining kangaroo care and maternal singing can be especially beneficial for mothers as it reduces their anxiety levels [106]. Here, the mother was instructed to sing with a repetitive, soothing tone, softly, simply, and with a slow tempo, i.e., the characteristics of infant-directed singing. In the preterm infants in the group exposed to kangaroo care and maternal singing, the authors observed better autonomic stability and a calming effect. During kangaroo care, the skin-to-skin contact between mother and preterm infant provides multisensory rhythmic stimulation in a unique, interactive way that can significantly decrease or mask the harmful effects of environmental stimuli. Roa and Ettenberger [107] studied kangaroo care using the rhythm, breath, and lullaby (RBL) model developed by Loewy [108] in order to replicate the auditory environment in the womb, such as slow tempo and repetition. With RBL, parents experience less anxiety, decreased stress levels, increased maternal relaxation, and more motivation [109,110] The music, the humming, and the vibration of a monochord placed on the kangaroo parent’s elbow so that the rhythmical vibrations can be felt by the preterm infant create a sense of closeness and intimacy, a new way of meeting and being together [111]. Kostilainen et al. [112] investigated the effects of daily singing combined with kangaroo carrying during the first weeks after preterm birth. Parents were encouraged to sing or hum at a slow tempo with repetitive and simple melodies during the kangaroo care for the time they liked. Parents who sang felt a positive impact on their well-being: singing improved interaction and made it easier for them to connect naturally with their child. They felt more relaxed when they sang, and they also felt that their child was more relaxed. Thus, singing during kangaroo care was mostly experienced as a shared, intimate moment between parent and infant. Can the multiplicity of rhythms created by skin-to-skin contact and the addition of a lullaby promote trans-natal continuity, potentially affording additional synchronization cues?
2. Empirical Pilot Study
2.1. Method
2.1.1. Participants
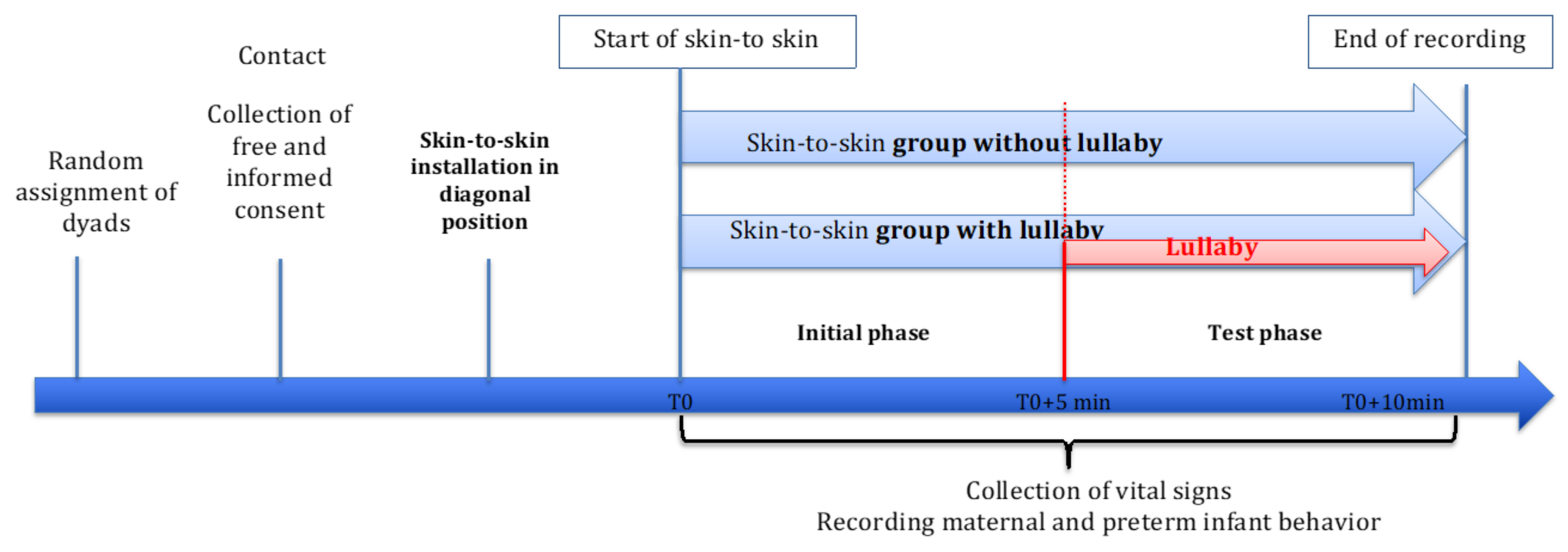
2.1.2. Procedure
2.1.3. Data Analysis
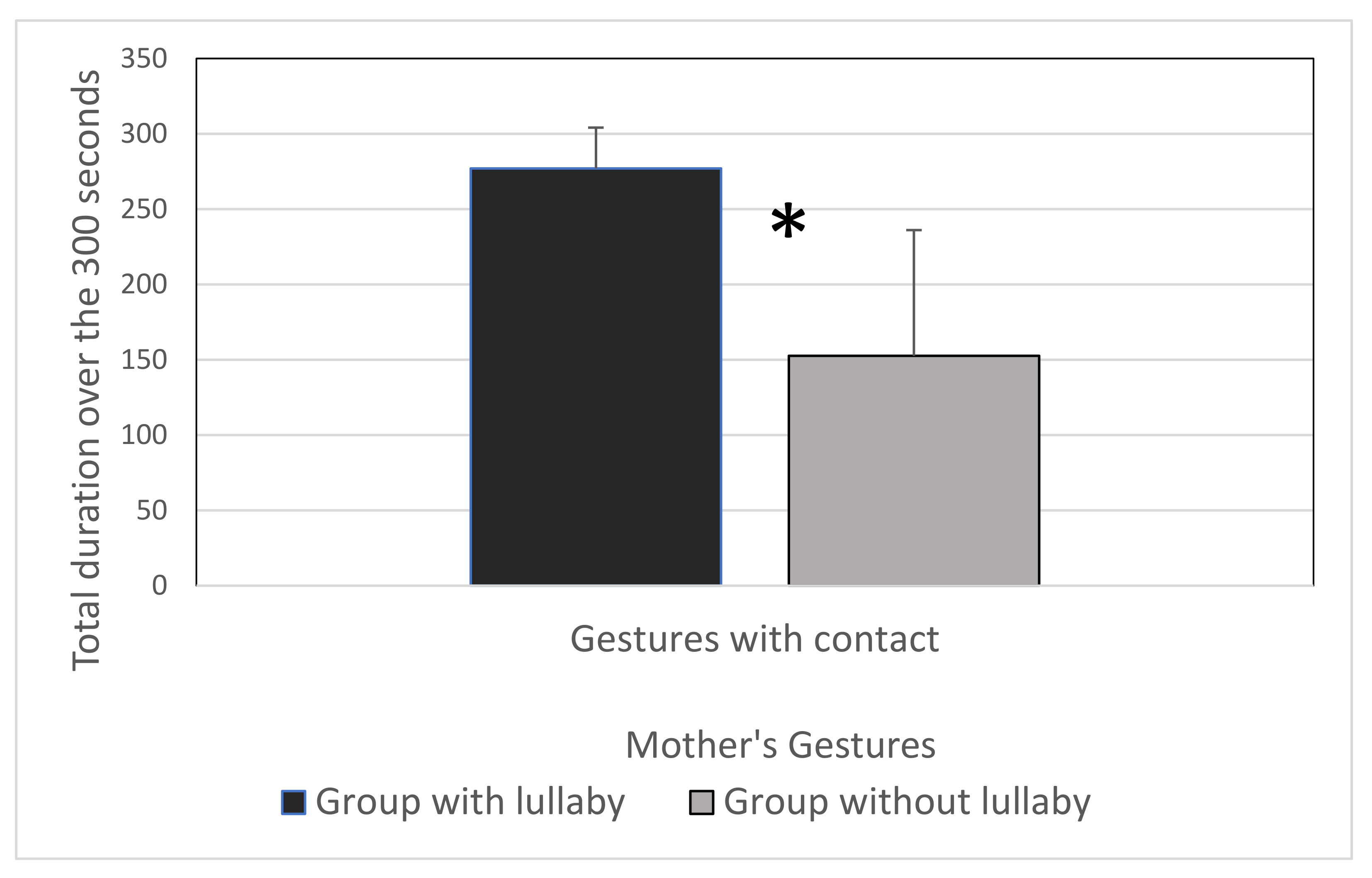
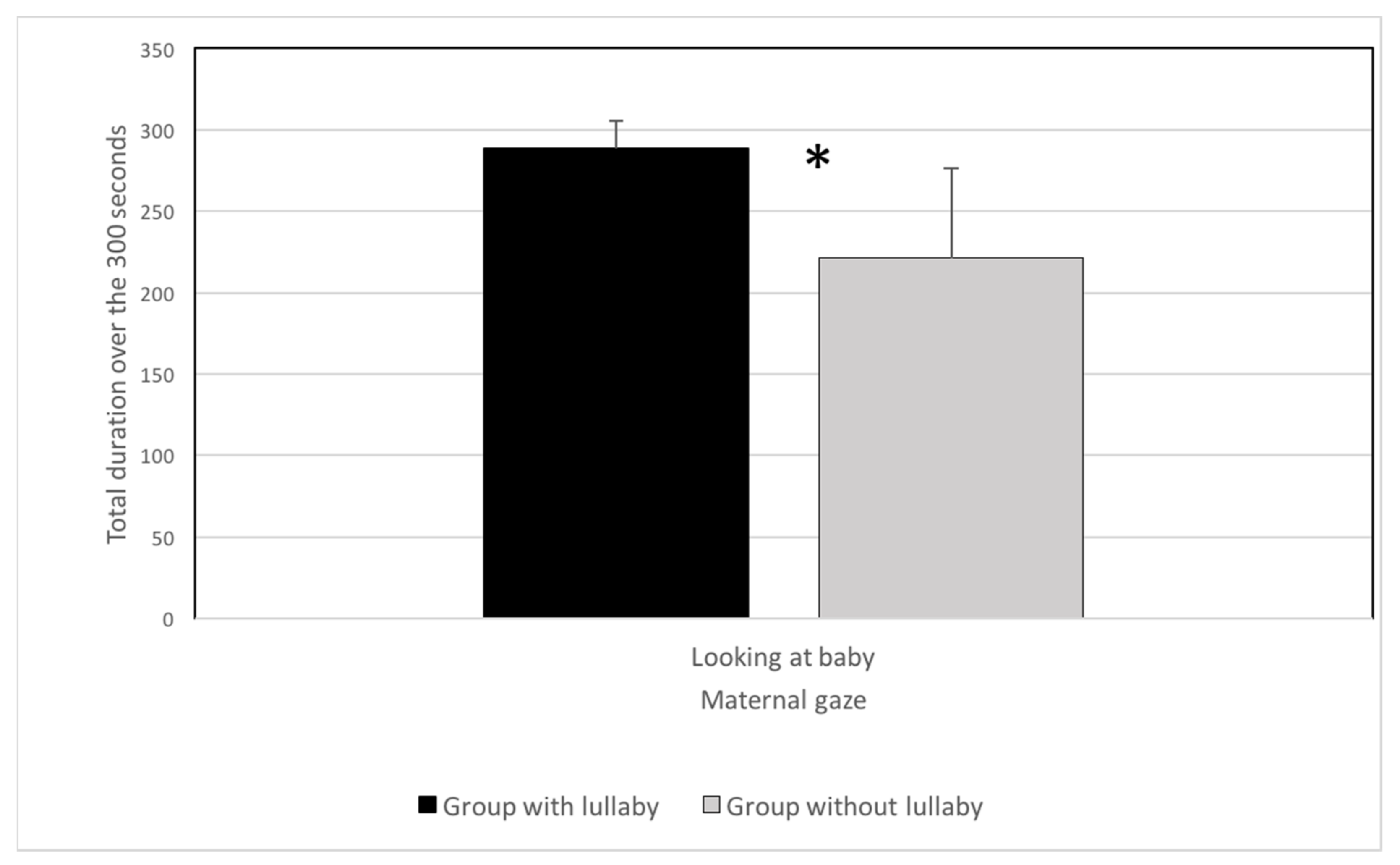
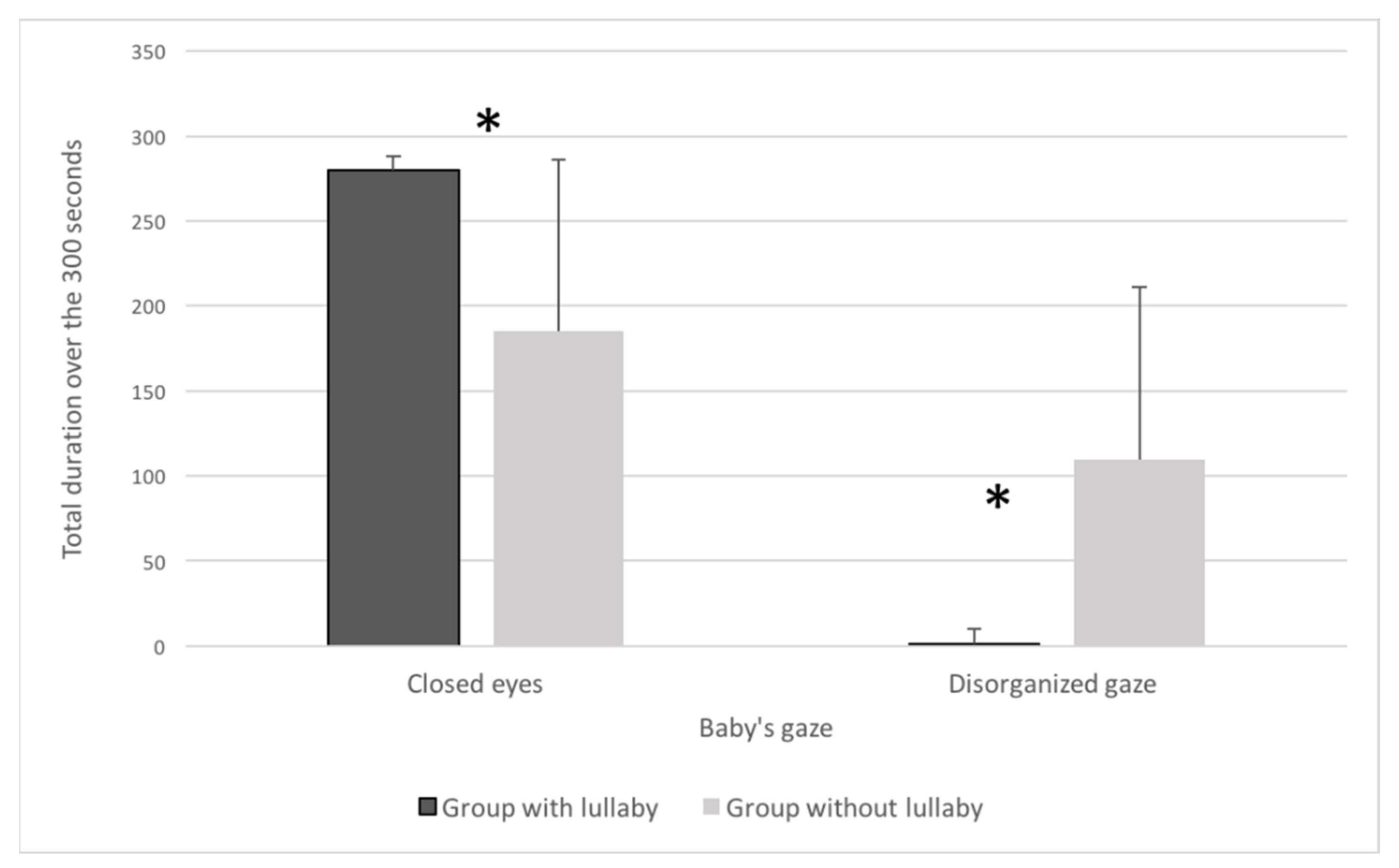
2.2. Results
2.3. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bonacina, S.; Krizman, J.; White-Schwoch, T.; Nicol, T.; Kraus, N. How rhythmic skills relate and develop in school-age children. Glob. Pediatric Health 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Provasi, J.; Anderson, D.I.; Barbu-Roth, M. Rhythm perception, production, and synchronization during the perinatal period. Front. Psychol. 2014, 5, 1048. [Google Scholar] [CrossRef] [Green Version]
- Iversen, J.R. In the beginning was the beat: Evolutionary origins of musical rhythm in humans. In The Cambridge Companion to Percussion; Hartenberger, R., Ed.; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Provasi, J. Comment le rythme vient aux bébés? Spirale 2016, 76, 50–63. [Google Scholar] [CrossRef]
- Parncutt, R. Prenatal development. In The Child as Musician: A Handbook of Musical Development; Oxford University Press: Oxford, UK, 2006; pp. 1–31. [Google Scholar]
- Graven, S.N.; Browne, J.V. Auditory development in the fetus and infant. Newborn Infant Nurs. Rev. 2008, 8, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Querleu, D.; Renard, X.; Versyp, F. Fetal hearing. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988, 28, 191–212. [Google Scholar] [CrossRef]
- Porcaro, C.; Zappasodi, F.; Barbati, G.; Salustri, C.; Pizzella, V.; Rossini, R.P.; Tecchio, F. Fetal auditory responses to external sounds and mother’s heartbeat: Detection improved by Independent Component Analysis. Brain Res. 2006, 1101, 51–58. [Google Scholar] [CrossRef]
- Monk, C.; Fifer, W.P.; Myers, M.M.; Sloan, R.P.; Trien, L.; Hurtado, A. Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Dev. Psychobiol. 2000, 36, 67–77. [Google Scholar] [CrossRef]
- Teie, D.A. Comparative analysis of the universal elements of music and the fetal environment. Front. Psychol. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullal-Gupta, S.; Vanden Bosch der Nederlanden, C.M.; Tichko, P.; Lahav, A.; Hannon, E.E. Linking prenatal experience to the emerging musical mind. Front. Syst. Neurosci. 2013, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Pino, O. Fetal memory: The effects of prenatal auditory experience on human development. BAOJ Med. Nurs. 2016, 2, 20. [Google Scholar] [CrossRef]
- Brackbill, Y.; Adams, G.; Crowell, D.H.; Gray, M.L. Arousal level in neonates and preschool children under continuous auditory stimulation. J. Exp. Child Psychol. 1966, 4, 178–188. [Google Scholar] [CrossRef]
- Tan, S.L.; Pfordresher, P.; Harré, R. Psychology of Music: From Sound to Significance; Psychology Press: London, UK, 2010. [Google Scholar] [CrossRef]
- Parncutt, R. Prenatal ‘experience’ and the phylogenesis and ontogenesis of music. In Music that Works. Contributions of Biology, Neurophysiology, Psychology, Sociology, Medicine and Musicology; Springer: Vienna, Austria, 2009; pp. 185–194. [Google Scholar] [CrossRef]
- Van Leeuwen, P.; Geue, D.; Thiel, M.; Cysarz, D.; Lange, S.; Romano, M.C.; Wessel, N.; Kurths, J.; Grönemeyer, D.H. Influence of paced maternal breathing on fetal-maternal heart rate coordination. Proc. Natl. Acad. Sci. USA 2009, 106, 13661–13666. [Google Scholar] [CrossRef] [Green Version]
- Philbin, K.M. The sound environments and auditory perceptions of the fetus and preterm newborn. In Early Vocal Contact and Preterm Infant Brain Development: Bridging the Gaps Between Research and Practice; Filippa, M., Westrup, B., Kuhn, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 91–111. [Google Scholar] [CrossRef] [Green Version]
- Lordier, L.; Loukas, S.; Grouiller, F.; Vollenweider, A.; Vasung, L.; Meskaldij, D.E.; Lejeune, F.; Pittet, M.-P.; Borradori-Tolsa, C.; Lazeyras, F.; et al. Music processing in preterm and full-term newborns: A psychophysiological interaction (PPI) approach in neonatal fMRI. Neuroimage 2019, 185, 857–864. [Google Scholar] [CrossRef]
- Masataka, N. The origins of language and the evolution of music: A comparative perspective. Phys. Life Rev. 2009, 6, 11–22. [Google Scholar] [CrossRef]
- Kisilevsky, S.; Hains, S.M.J.; Jacquet, A.Y.; Granier-Deferre, C.; Lecanuet, J.P. Maturation of fetal responses to music. Dev. Sci. 2004, 7, 550–559. [Google Scholar] [CrossRef]
- Wöllner, C.; Hammerschmidt, D. In sync with hip-hop: Effects of cognitive load, arousal, and musical meter on perceived time. Atten. Percept. Psychophys. 2020, 36, 1–17. [Google Scholar]
- Kisilevsky, B.; Hains, S.; Lee, K. Effects of experience on fetal voice recognition. Psychol. Sci. 2003, 14, 220–224. [Google Scholar] [CrossRef]
- Kisilevsky, B.S.; Hains, S.M.J.; Brown, C.A.; Lee, C.T.; Cowperthwaite, B.; Stutzman, S.S.; Swansburg, M.L.; Lee, K.; Xie, X.; Huang, H.; et al. Fetal sensitivity to properties of maternal speech and language. Infant Behav. Dev. 2009, 32, 59–71. [Google Scholar] [CrossRef]
- Nazzi, T.; Bertoncini, J.; Mehler, J. Language discrimination by newborns: Toward an understanding of the role of rhythm. J. Exp. Psychol. Hum. Percept. Perform. 1998, 24, 756–766. [Google Scholar] [CrossRef]
- Háden, G.P.; Honing, H.; Török, M.; Winkler, I. Detecting the temporal structure of sound sequences in newborn infants. Int. J. Psychophysiol. 2015, 96, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; De Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough? For adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Fell, D.B.; Joseph, K.S.; Armson, B.A.; Dodds, L. The impact of pregnancy on physical activity level. Matern. Child Health J. 2009, 13, 597–603. [Google Scholar] [CrossRef]
- Chandonnet, N.; Saey, D.; Alméras, N.; Marc, I. French pregnancy physical activity questionnaire compared with an accelerometer cut point to classify physical activity among pregnant obese women. PLoS ONE 2012, 7, e38818. [Google Scholar] [CrossRef] [Green Version]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Polańska, K.; Muszyński, P.; Sobala, W.; Dziewirska, E.; Merecz-Kot, D.; Hanke, W. Maternal lifestyle during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Early Hum. Dev. 2015, 91, 317–325. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Schmidt, M.D.; Roberts, D.E.; Hosmer, D.; Markenson, G.; Freedson, P.S. Development and validation of a pregnancy physical activity questionnaire. Med. Sci. Sports Exerc. 2004, 36, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Lecanuet, J.P.; Schaal, B. Sensory performances in the human foetus: A brief summary of research. Intellectica 2002, 1, 29–56. [Google Scholar] [CrossRef] [Green Version]
- Porton-Deterne, I.; Le Du, A.; Jacquet, A.Y.; Lecanuet, J.P. Réactivité du foetus de fin de gestation a des pressions transabdominales en fonction de l’état de vigilance. Neuropsychiatr. Enfance. Adolesc. 1997, 45, 700–704. [Google Scholar]
- Lecanuet, J.P.; Jacquet, A.Y. Fetal responsiveness to maternal passive swinging in low heart rate variability state: Effects of stimulation direction and duration. Dev. Psychobiol. 2002, 40, 57–67. [Google Scholar] [CrossRef]
- Cito, G.; Luisi, S.; Mezzesimi, A.; Cavicchioli, C.; Calonaci, G.; Petraglia, F. Maternal position during non-stress test and fetal heart rate patterns: Editorial comment. Acta Obstet. Gynecol. Scand. 2005, 60, 500–501. [Google Scholar]
- Granier-Deferre, C.; Bassereau, S.; Ribeiro, A.; Jacquet, A.-Y.; Decasper, A.J. A melodic contour repeatedly experienced by human near-term fetuses elicits a profound cardiac reaction one month after birth. PLoS ONE 2011, 6, e17304. [Google Scholar] [CrossRef] [Green Version]
- Vinall, J.; Riddell, R.P.; Greenberg, S. The influence of culture on maternal soothing behaviours and infant pain expression in the immunization context. Pain Res. Manag. 2011, 16, 234–238. [Google Scholar] [CrossRef]
- Richter, J.; Ostovar, R. “It don’t mean a thing if it ain’t got that swing”—An alternative concept for understanding the evolution of dance and music in human beings. Front. Hum. Neurosci. 2016, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lickliter, R.; Bahrick, L.E. Using an animal model to explore the prenatal origins of social development. In Fetal Development: Research on Brain and Behavior, Environmental Influences, and Emerging Technologie; Reissland, N., Kisilevsky, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Lickliter, R.; Bahrick, L.E. The salience of multimodal sensory stimulation in early development: Implications for the issue of ecological validity. Infancy 2001, 2, 451–467. [Google Scholar] [CrossRef]
- Lickliter, R. The influence of prenatal experience on behavioral and social development: The benefits and limitations of an animal model. Dev. Psychopathol. 2018, 30, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Changizi, M. Harnessed: How Language and Music Mimicked Nature and Transformed Ape to Man; BenBella Books, Inc: Dallas, TX, USA, 2011. [Google Scholar]
- Provasi, J.; Granier-Deferre, C. Apprentissages et mémoire au cours de la période périnatale. In Le Développement du Bébé de la vie Foetale à la Marche; Devouche, E., Provasi, J., Eds.; Elsevier Masson: Paris, France, 2019; pp. 43–58. [Google Scholar] [CrossRef]
- Fraisse, P. Traitement d’informations successives dans la reconnaissance de l’identité de noms et de dessins. Année Psychol. 1974, 74, 403–417. [Google Scholar] [CrossRef]
- Ravignani, A.; Dalla Bella, S.; Falk, S.; Kello, C.T.; Noriega, F.; Kotz, S.A. Rhythm in speech and animal vocalizations: A cross-species perspective. Ann. N. Y. Acad. Sci. 2019, 1453, 79–98. [Google Scholar] [CrossRef] [Green Version]
- Honing, H. Without it no music: Beat induction as a fundamental musical trait. Ann. N. Y. Acad. Sci. 2012, 1252, 85–91. [Google Scholar] [CrossRef]
- Gingras, J.L.; Mitchell, E.A.; Grattan, K.E. Fetal homologue of infant crying. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F415–F418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravignani, A.; Dalla Bella, S.; Falk, S.; Kello, C.; Noriega, F.; Kotz, S.A. Evolution of speech rhythm: A cross-species perspective. PeerJ Prepr. 2019. [Google Scholar] [CrossRef]
- Condon, W.; Sander, L. Neonate movement is synchronized with adult speech: Interactional participation and language acquisition. Science 1974, 74, 99–101. [Google Scholar] [CrossRef]
- Dominguez, S.; Devouche, E.; Apter, G.; Gratier, M. The roots of turn-taking in the neonatal period. Infant Child Dev. 2016, 25, 240–255. [Google Scholar] [CrossRef]
- Schachner, A. The origins of human and avian auditory-motor entrainment. Nova Acta Leopold. 2013, 111, 243–253. [Google Scholar]
- Bobin-Bègue, A. Le tempo, fondement des compétences musicales et support du développement sociocognitif. Enfance 2020, 5, 109–129. [Google Scholar] [CrossRef]
- Bobin-Bègue, A. Rhythms in early development. In Early Interaction and Developmental Psychopathology; Apter, G., Devouche, E., Gratier, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 55–86. [Google Scholar] [CrossRef]
- Zimmerman, E.; Barlow, S.M. The effects of vestibular stimulation rate and magnitude of acceleration on central pattern generation for chest wall kinematics in preterm infants. J. Perinatol. 2012, 32, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Lahav, A.; Skoe, E. An acoustic gap between the NICU and womb: A potential risk for compromised neuroplasticity of the auditory system in preterm infants. Front. Neurosci. 2014, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caskey, M.; Vohr, B. Assessing language and language environment of high-risk infants and children: A new approach. Acta Paediatr. 2013, 102, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Hatcher, R.P.; Barglow, P.D. Prematurity and infant stimulation: A review of research. Child Psychiatry Hum. Dev. 1980, 10, 199–212. [Google Scholar] [CrossRef]
- Korner, A.F. Infant stimulation. Issues of theory and research. Clin. Perinatol. 1990, 17, 173–184. [Google Scholar] [CrossRef]
- Cramer, S.J.E.; Dekker, J.; Dankelman, J.; Pauws, S.C.; Hooper, S.B.; Te Pas, A.B. Effect of tactile stimulation on termination and prevention of apnea of prematurity: A systematic review. Front. Pediatrics 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Korner, A.F.; Schneider, P.; Forrest, T. Effects of vestibular-proprioceptive stimulation on the neurobehavioral development of preterm infants: A pilot study. Neuropediatrics 1983, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Korner, A.F.; Guilleminault, C.; Van Den Hoed, J.; Baldwin, R.B. Reduction of sleep apnea and bradycardia in preterm infants on oscillating water beds: A controlled polygraphic study. Pediatrics 1978, 61, 528–533. [Google Scholar] [PubMed]
- Groswasser, J.; Sottiaux, M.; Rebuffat, E. Reduction in obstructive breathing events during body rocking: A controlled polygraphic study in preterm and full-term infants. Pediatrics 1995, 96, 64–68. [Google Scholar] [PubMed]
- Jones, R.A.K. A controlled trial of a regularly cycled oscillating waterbed and a non-oscillating waterbed in the prevention of apnoea in the preterm infant. Arch. Dis. Child. 1981, 56, 889–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigal, S.; Watts, J.; Campbell, D. Randomized clinical trial of an oscillating air mattress in preterm infants: Effect on apnea, growth, and development. J. Pediatrics 1986, 109, 857–864. [Google Scholar] [CrossRef]
- Freedman, D.; Boverman, H.; Freedman, N. Effects of kinesthetic stimulation on certain aspects of development in premature infants. Presented at the Meeting of the American Orthopsychiatric Association, San Francisco, CA, USA, 29–30 April 1966. Unpublished Paper. [Google Scholar]
- Clark, D.L.; Cordero, L.; Goss, K.C.; Manos, D. Effects of rocking on neuromuscular development in the premature. Biol. Neonate 1989, 56, 306–314. [Google Scholar] [CrossRef]
- Sammon, M.P.; Darnall, R.A. Entrainment of respiration to rocking in premature infants: Coherence analysis. J. Appl. Physiol. 1994, 77, 1548–1554. [Google Scholar] [CrossRef]
- Tuck, S.J.; Monin, P.; Duvivier, C.; May, T.; Vert, P. Effect of a rocking bed on apnoea of prematurity. Arch. Dis. Child. 1982, 57, 475–477. [Google Scholar] [CrossRef] [Green Version]
- Barlow, S.M.; Finan, D.S.; Lee, J.; Chu, S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J. Perinatol. 2008, 28, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Perrault, A.A.; Khani, A.; Quairiaux, C.; Kompotis, K.; Franken, P.; Muhlethaler, M.; Schwartz, S.; Bayer, L. Whole-night continuous rocking entrains spontaneous neural oscillations with benefits for sleep and memory. Curr. Biol. 2019, 29, 402–411.e3. [Google Scholar] [CrossRef] [Green Version]
- Thoman, E.B.; Ingersoll, E.W.; Acebo, C. Premature infants seek rhythmic stimulation, and the experience facilitates neurobehavioral development. J. Dev. Behav. Pediatrics 1991, 12, 11–18. [Google Scholar] [CrossRef]
- Thoman, E.B.; Graham, S.E. Self-regulation of stimulation by premature infants. Pediatrics 1986, 78, 855–860. [Google Scholar]
- Song, D.; Jegatheesan, P.; Nafday, S.; Ahmad, K.A.; Nedrelow, J.; Wearden, M.; Nemerofsky, S.; Pooley, S.; Thompson, D.; Vail, D.; et al. Patterned frequency-modulated oral stimulation in preterm infants: A multicenter randomized controlled trial. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.I.; Pierpont, M.E. Rocking waterbeds and auditory stimuli to enhance growth of preterm infants. Preliminary report. J. Pediatrics 1976, 88, 297–299. [Google Scholar] [CrossRef]
- Barnard, K.E.; Bee, H.L. The impact of temporally patterned stimulation on the development of preterm infants. Child Dev. 1983, 54, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Barnard, K. The effect of stimulation on the sleep behavior of the premature infant. Commun. Nurs. Res. 1973, 6, 12–33. [Google Scholar]
- Rice, R.D. Neurophysiological development in premature infants following stimulation. Dev. Psychol. 1977, 13, 69–76. [Google Scholar] [CrossRef]
- Burns, K.; Cunningham, N.; White-Traut, R.; Silvestri, J.; Nelson, M. Infant stimulation: Modification of an intervention based on physiologic and behavioral cues. J. Obstet. Gynecol. Neonatal Nurs. 1994, 23, 581–589. [Google Scholar] [CrossRef] [PubMed]
- White-Traut, R.; Norr, K.F.; Fabiyi, C.; Rankin, K.M.; Li, Z.; Liu, L. Mother-infant interaction improves with a developmental intervention for mother-preterm infant dyads. Infant Behav. Dev. 2013, 36, 694–706. [Google Scholar] [CrossRef] [Green Version]
- White-Traut, R. Providing a nurturing environment for infants in adverse situations: Multisensory strategies for newborn care. J. Midwifery Women’s Health 2004, 49, 36–41. [Google Scholar] [CrossRef]
- Holditch-Davis, D.; Santos, H.; Levy, J.; White-Traut, R.C.; O’Shea, T.M.; Geraldo, V.; David, R. Patterns of psychological distress in mothers of preterm infants. Infant Behav. Dev. 2015, 41, 154–163. [Google Scholar] [CrossRef] [Green Version]
- White-Traut, R.; Wink, T.; Minehart, T.; Holditch-Davis, D. Frequency of premature infant engagement and disengagement behaviors during two maternally administered interventions. Newborn Infant Nurs. Rev. 2012, 12, 124–131. [Google Scholar] [CrossRef] [Green Version]
- White-Traut, R.C.; Nelson, M.N. Maternally administered tactile, auditory, visual, and vestibular stimulation: Relationship to later interactions between mothers and premature infants. Res. Nurs. Health 1988, 11, 31–39. [Google Scholar] [CrossRef]
- White-Traut, R.C.; Nelson, M.N.; Silvestri, J.M.; Vasan, U.; Littau, S.; Meleedy-Rey, P.; Gu, G.; Patel, M. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev. Med. Child Neurol. 2002, 44, 91–97. [Google Scholar] [CrossRef]
- Holditch-Davis, D.; White-Traut, R.C.; Levy, J.A.; O’Shea, T.M.; Geraldo, V.; David, R.J. Maternally administered interventions for preterm infants in the NICU: Effects on maternal psychological distress and mother-infant relationship. Infant Behav. Dev. 2014, 37, 695–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holditch-Davis, D.; White-Traut, R.; Levy, J.; Williams, K.L.; Ryan, D.; Vonderheid, S. Maternal satisfaction with administering infant interventions in the NICU. J. Obs. Gynecol. Neonatal Nurs. 2013, 42, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White-Traut, R.C.; Nelson, M.N.; Silvestri, J.M.; Cunningham, N.; Patel, M. Responses of preterm infants to unimodal and multimodal sensory intervention. Pediatric Nurs. 1997, 23, 169–177. [Google Scholar]
- Pineda, R.; Wallendorf, M.; Smith, J. A pilot study demonstrating the impact of the supporting and enhancing NICU sensory experiences (SENSE) program on the mother and infant. Early Hum. Dev. 2020, 144, 105000. [Google Scholar] [CrossRef]
- Doheny, L.; Hurwitz, S.; Insoft, R.; Ringer, S.; Lahav, A. Exposure to biological maternal sounds improves cardiorespiratory regulation in extremely preterm infants. J. Matern. Neonatal Med. 2012, 25, 1591–1594. [Google Scholar] [CrossRef]
- Webb, A.R.; Heller, H.T.; Benson, C.B.; Lahav, A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc. Natl. Acad. Sci. USA 2015, 112, 3152–3157. [Google Scholar] [CrossRef] [Green Version]
- Filippa, M.; Panza, C.; Ferrari, F.; Frassoldati, R.; Kuhn, P.; Balduzzi, S.; D’Amico, R. Systematic review of maternal voice interventions demonstrates increased stability in preterm infants. Acta Paediatr. 2017, 1–10. [Google Scholar] [CrossRef]
- Rickard, N.S.; Toukhsati, S.R.; Field, S.E. The effect of music on cognitive performance: Insight from neurobiological and animal studies. Behav. Cogn. Neurosci. Rev. 2005, 4, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, F.B. Music therapy for premature infants and their parents: An integrative review. Nord. J. Music Ther. 2012, 21, 203–226. [Google Scholar] [CrossRef]
- Robb, S.L.; Hanson-Abromeit, D.; May, L.; Hernandez-Ruiz, E.; Allison, M.; Beloat, A.; Daugherty, S.; Kurtz, R.; Ott, A.; Oladimeji Oyedele, O.; et al. Reporting quality of music intervention research in healthcare: A systematic review. Complementary Ther. Med. 2018, 38, 24–41. [Google Scholar] [CrossRef]
- Lordier, L.; Meskaldjia, D.E.; Grouillerd, F.; Pitteta, M.P.; Vollenweidera, A.; Vasunga, L.; Borradori-Tolsaa, C.; Lazeyrase, F.; Grandjean, D.; Van De Ville, D.; et al. Music in premature infants enhances high-level cognitive brain networks. Proc. Natl. Acad. Sci. USA 2019, 116, 12103–12108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rand, K.; Lahav, A. Maternal sounds elicit lower heart rate in preterm newborns in the first month of life. Early Hum. Dev. 2014, 90, 679–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malloch, S.; Shoemark, H.; Crncec, R.; Newnham, C.; Paul, C.; Prior, M.; Coward, S.; Burnham, D. Music therapy with hospitalized infants-the art and science of communicative musicality. Infant Ment. Health J. 2012, 33, 386–399. [Google Scholar] [CrossRef]
- Bieleninik, L.; Ghetti, C.; Gold, C. Music therapy for preterm infants and their parents: A meta-analysis. Pediatrics 2016, 138, e20160971. [Google Scholar] [CrossRef] [Green Version]
- Standley, J.M. The effect of contingent music to increase non-nutritive sucking of premature infants. Pediatric Nurs. 2000, 26, 43. [Google Scholar]
- Standley, J.M. The effect of music-reinforced nonnutritive sucking on feeding rate of premature infants. J. Pediatric Nurs. 2003, 18, 169–173. [Google Scholar] [CrossRef]
- Standley, J.M.; Cassidy, J.; Grant, R.; Cevasco, A.; Szuch, C.; Nguyen, J.; Walworth, D.; Procelli, D.; Jarred, J.; Adams, K. The effect of music reinforcement for non-nutritive sucking on nipple feeding of premature infants. Pediatric Nurs. 2010, 36, 138–145. [Google Scholar]
- Bigelow, A.E.; Power, M. Mother–infant skin-to-skin contact: Short- and long-term effects for mothers and their children born full-term. Front. Psychol. 2020, 11, 1921. [Google Scholar] [CrossRef]
- Cooper, R.P.; Aslin, R.N. The language environment of the young infant: Implications for early perceptual development. Can. J. Psychol. 1989, 43, 247–265. [Google Scholar] [CrossRef]
- Trehub, S.E.; Unyk, A.M.; Kamenetsky, S.B.; Hill, D.S.; Trainor, L.J.; Henderson, J.L.; Saraza, M. Mothers’ and fathers’ singing to infants. Dev. Psychol. 1997, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Weller, A.; Sirota, L.; Eidelman, A.I. Skin-to-Skin contact (Kangaroo care) promotes self-regulation in premature infants: Sleep-wake cyclicity, arousal modulation, and sustained exploration. Dev. Psychol. 2002, 38, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.; Diamant, C.; Bauer, S.; Regev, R.; Sirota, G.; Litmanovitz, I. Department Maternal singing during kangaroo care led to autonomic stability in preterm infants and reduced maternal anxiety. Acta Paediatr. 2014, 103, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Roa, E.; Ettenberger, M. Music therapy self-care group for parents of preterm infants in the neonatal intensive care unit: A clinical pilot intervention. Medicines 2018, 5, 134. [Google Scholar] [CrossRef] [Green Version]
- Loewy, J. NICU music therapy: Song of kin as critical lullaby in research and practice. Ann. N. Y. Acad. Sci. 2015, 1337, 178–185. [Google Scholar] [CrossRef]
- Ettenberger, M. Music therapy in the neonatal intensive care unit: Putting the families at the center of care. Br. J. Music Ther. 2017, 31, 12–17. [Google Scholar] [CrossRef]
- Ettenberger, M.; Odell-Miller, H.; Rojas Cárdenas, C.; Torres Serrano, S.; Parker, M.; Camargo Llanos, S.M. Music therapy with premature infants and their caregivers in Colombia—A mixed methods pilot study including a randomized trial. Voices A World Forum Music Ther. 2014, 14, 1–28. [Google Scholar] [CrossRef]
- Kehl, S.M.; La Marca-Ghaemmaghami, P.; Haller, M.; Pichler-Stachl, E.; Bucher, H.U.; Bassler, D.; Haslbeck, F.B. Creative music therapy with premature infants and their parents: A mixed-method pilot study on parents’ anxiety, stress and depressive symptoms and parent—Infant attachment. Int. J. Environ. Res. Public Health 2021, 18, 265. [Google Scholar]
- Kostilainen, K.; Mikkola, K.; Erkkilä, J.; Huotilainen, M. Effects of maternal singing during kangaroo care on maternal anxiety, wellbeing, and mother-infant relationship after preterm birth: A mixed methods study. Nord. J. Music Ther. 2020, 30, 1–20. [Google Scholar]
- Prechtl, H.F.R. The behavioural states of the newborn infant (a review). Brain Res. 1974, 76, 185–212. [Google Scholar] [CrossRef]
- Buil, A.; Carchon, I.; Apter, G.; Laborne, F.X.; Granier, M.; Devouche, E. Kangaroo supported diagonal flexion positioning: New insights into skin-to-skin contact for communication between mothers and very preterm infants. Archives de Pédiatrie 2016, 23, 913–920. [Google Scholar] [CrossRef]
- Croy, I.; Luong, A.; Triscoli, C.; Hofmann, E.; Olausson, H.; Sailer, U. Interpersonal stroking touch is targeted to C tactile afferent activation. Behav. Brain Res. 2016, 297, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Van Puyvelde, M.; Gorissen, A.-S.; Pattyn, N.; McGlone, F.P. Does touch matter? The impact of stroking versus non-stroking maternal touch on cardio-respiratory processes in mothers and infants. Physiol. Behav. 2019, 207, 55–63. [Google Scholar] [CrossRef]
- Manzotti, A.; Cerritellia, F.; Esteves, J.E.; Lista, G.; Lombardi, E.; La Rocca, S.; Gallace, A.; McGlone, F.P.; Walker, S.C. Dynamic touch reduces physiological arousal in preterm infants: A role for c-tactile afferents? Dev. Cogn. Neurosci. 2019, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brovetto, E. La chanson: Dans le lien primaire mère-bébé et dans le domaine thérapeutique. La berceuse. In Collection 1001 Bébés; Érès: Toulouse, France, 2008; pp. 127–151. [Google Scholar] [CrossRef]
- Nadel, J.; Carchon, I.; Kervella, C.; Marcelli, D.; Râ, D. Expectancies for social contingency in 2-month-olds. Dev. Sci. 1999, 2, 164–173. [Google Scholar] [CrossRef]
- Gottlieb, G. Experiential canalization of behavioral development: Results. Dev. Psychol. 1991, 27, 35–39. [Google Scholar] [CrossRef]
- Markova, G.; Nguyen, T.; Hoehl, S. Neurobehavioral interpersonal synchrony in early development: The role of interactional rhythms. Front. Psychol. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Trevarthen, C. The musical art of infant conversation: Narrating in the time of sympathetic experience, without rational interpretation, before words. Musicae Sci. 2008, 15–46. [Google Scholar] [CrossRef]
- Provasi, J. Parent-Preterm Infant Interaction. In Early Interaction and Developmental Psychopathology; Apter, G., Devouche, E., Gratier, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 123–149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provasi, J.; Blanc, L.; Carchon, I. The Importance of Rhythmic Stimulation for Preterm Infants in the NICU. Children 2021, 8, 660. https://doi.org/10.3390/children8080660
Provasi J, Blanc L, Carchon I. The Importance of Rhythmic Stimulation for Preterm Infants in the NICU. Children. 2021; 8(8):660. https://doi.org/10.3390/children8080660
Chicago/Turabian StyleProvasi, Joëlle, Loreline Blanc, and Isabelle Carchon. 2021. "The Importance of Rhythmic Stimulation for Preterm Infants in the NICU" Children 8, no. 8: 660. https://doi.org/10.3390/children8080660
APA StyleProvasi, J., Blanc, L., & Carchon, I. (2021). The Importance of Rhythmic Stimulation for Preterm Infants in the NICU. Children, 8(8), 660. https://doi.org/10.3390/children8080660




