Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves
Abstract
:1. Introduction
2. Methods
2.1. Measurement Setup and Equipment
2.2. Acoustic Properties of the Incubator
2.3. Transfer of Noise into the Incubator
2.4. Characterization of the Environmental Noise at the NICU
2.5. Data Recording and Processing
3. Results
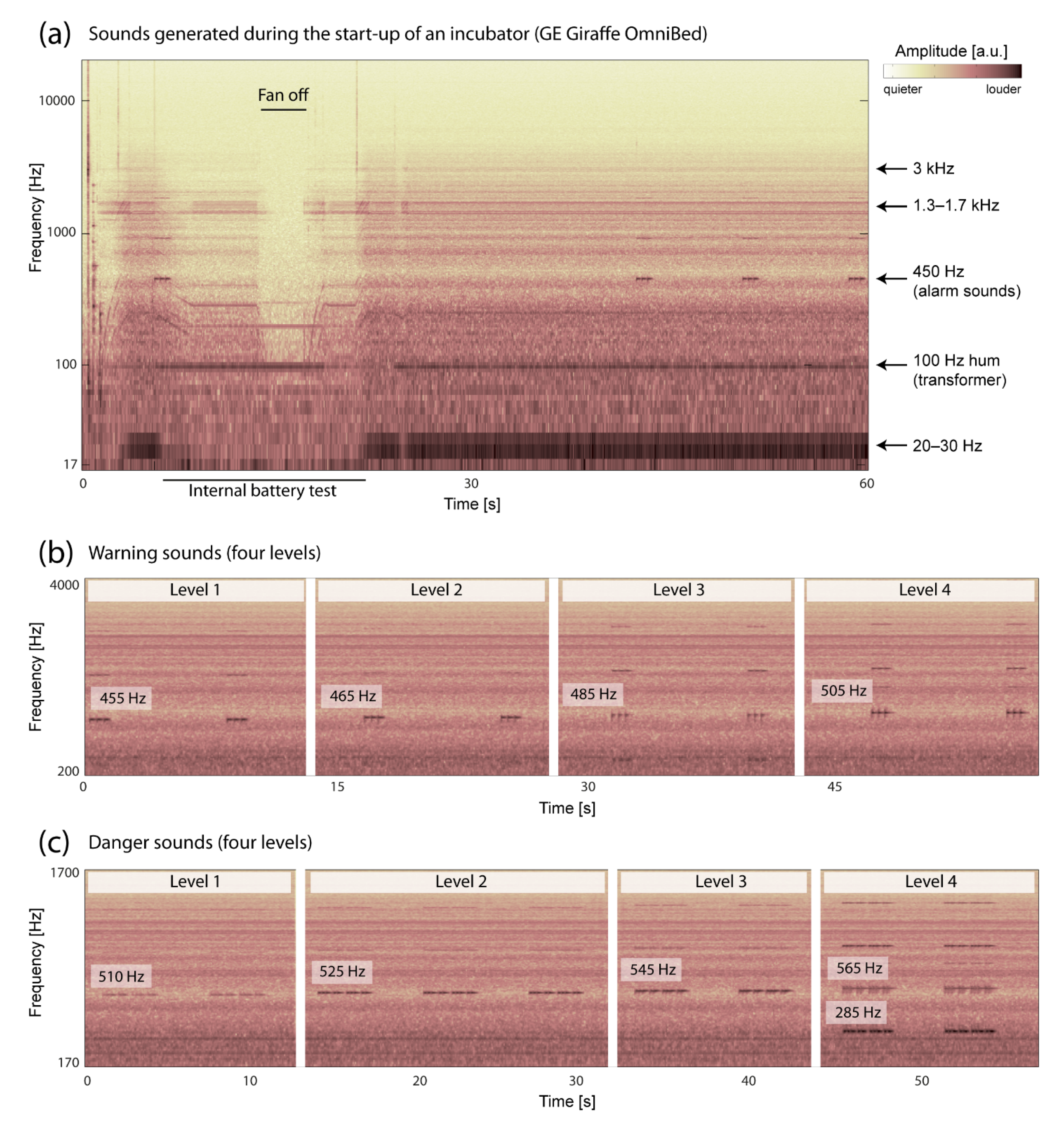
3.1. Acoustic Properties of the Incubator
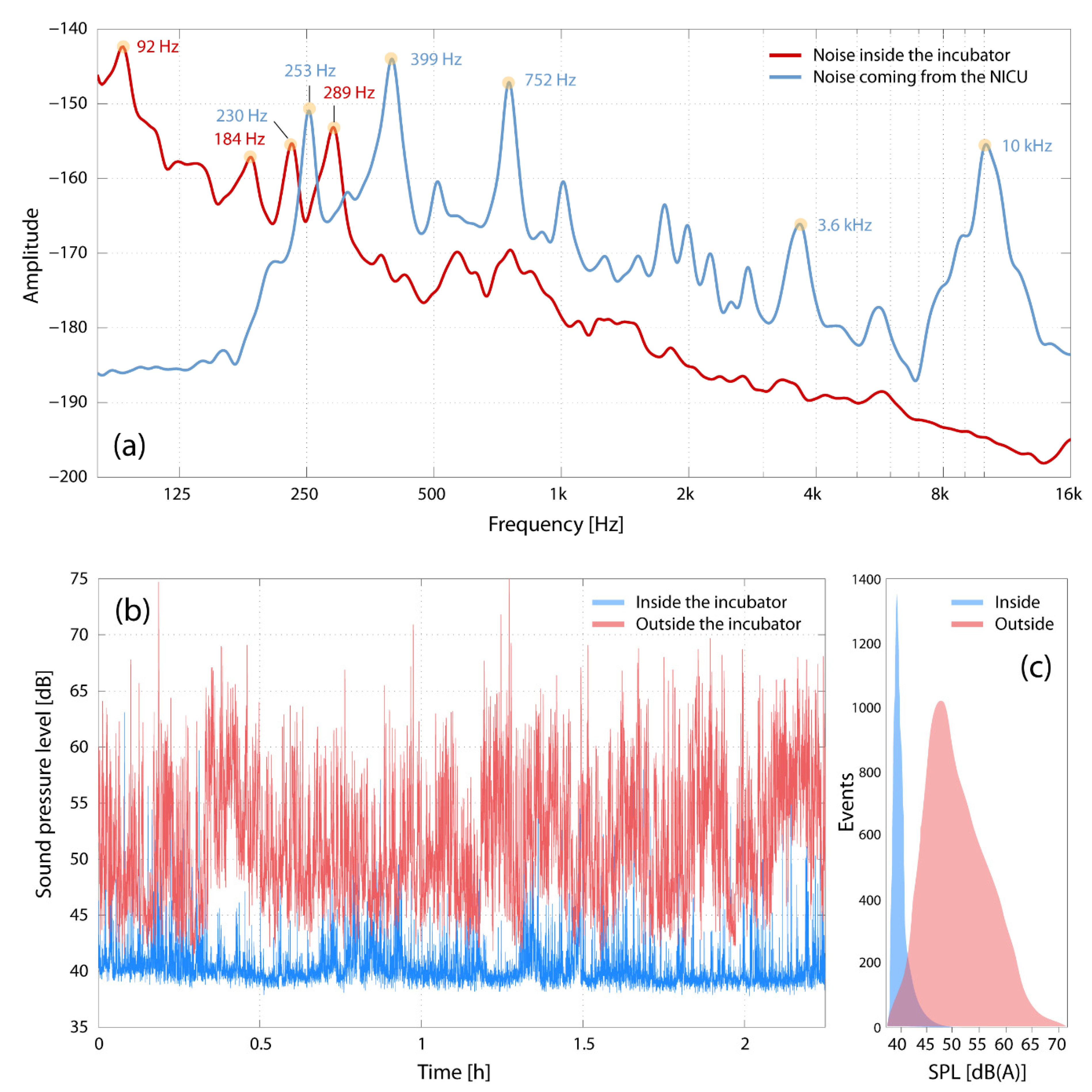
3.2. Transfer of Noise into the Incubator
3.3. Characterization of the Environmental Noise at the NICU
4. Discussion, Conclusions, and Outlook
4.1. Acoustic Properties of the Incubator
4.2. Transfer of Noise into the Incubator
4.3. Characterization of Environmental Noise at the NICU
4.4. Strengths and Limitations of This Study
4.5. Conclusions for the Newborn inside the Incubator
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Kuhn, P.; Zores, C.; Langlet, C.; Escande, B.; Astruc, D.; Dufour, A. Moderate acoustic changes can disrupt the sleep of very preterm infants in their incubators. Acta Paediatr. 2013, 102, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska-Seniuk, K.; Greczka, G.; Dabrowski, P.; Szyfter-Harris, J.; Mazela, J. Hearing impairment in premature newborns-Analysis based on the national hearing screening database in Poland. PLoS ONE 2017, 12, e0184359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlman, J.M. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics 2001, 108, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.F. Environmental Noise Retards Auditory Cortical Development. Science 2003, 300, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Pineda, R.G.; Neil, J.; Dierker, D.; Smyser, C.D.; Wallendorf, M.; Kidokoro, H.; Reynolds, L.C.; Walker, S.; Rogers, C.; Mathur, A.M.; et al. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. J. Pediatr. 2014, 164, 52–60.e2. [Google Scholar] [CrossRef] [Green Version]
- Haslbeck, F.B.; Jakab, A.; Held, U.; Bassler, D.; Bucher, H.U.; Hagmann, C. Creative music therapy to promote brain function and brain structure in preterm infants: A randomized controlled pilot study. Neuroimage Clin. 2020, 25, 102171. [Google Scholar] [CrossRef]
- Caskey, M.; Stephens, B.; Tucker, R.; Vohr, B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics 2011, 128, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Abrams, R.M.; Gerhardt, K.J. The acoustic environment and physiological responses of the fetus. J. Perinatol. 2000, 20, S31–S36. [Google Scholar] [CrossRef]
- Hepper, P.G.; Shahidullah, B.S. Development of fetal hearing. Arch. Dis. Child. Fetal. Neonatal. Ed. 1994, 71, F81–F87. [Google Scholar] [CrossRef] [Green Version]
- ACOG Committee on Obstetric Practice. Guidelines for Perinatal Care, 7th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2012. [Google Scholar]
- Levy, G.D.; Woolston, D.J.; Browne, J.V. Mean noise amounts in level II vs level III neonatal intensive care units. Neonatal. Netw. 2003, 22, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mankin, R.W. Acoustical detection of Aedes taeniorhynchus swarms and emergence exoduses in remote salt marshes. J. Am. Mosq. Control. Assoc. 1994, 10, 302–308. [Google Scholar]
- Almadhoob, A.; Ohlsson, A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2020, 1, CD010333. [Google Scholar] [CrossRef]
- Thomas, K.A.; Martin, P.A. NICU sound environment and the potential problems for caregivers. J. Perinatol. 2000, 20, S94–S99. [Google Scholar] [CrossRef] [Green Version]
- Verderber, S.; Gray, S.; Suresh-Kumar, S.; Kercz, D.; Parshuram, C. Intensive Care Unit Built Environments: A Comprehensive Literature Review (2005–2020). HERD 2021. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Leger, D.; Sauvet, F.; Champigneulle, B.; Rio, S.; Strauss, M.; Chennaoui, M.; Guilleminault, C.; Mira, J.P. Sound level intensity severely disrupts sleep in ventilated ICU patients throughout a 24-h period: A preliminary 24-h study of sleep stages and associated sound levels. Ann. Intensive Care 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bry, A.; Wigert, H. Psychosocial support for parents of extremely preterm infants in neonatal intensive care: A qualitative interview study. BMC Psychol. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.; Du, L.; Ahmad, K. Incubator-based Sound Attenuation: Active Noise Control In A Simulated Clinical Environment. PLoS ONE 2020, 15, e0235287. [Google Scholar] [CrossRef]
- Pineda, R.; Guth, R.; Herring, A.; Reynolds, L.; Oberle, S.; Smith, J. Enhancing sensory experiences for very preterm infants in the NICU: An integrative review. J. Perinatol. 2017, 37, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Filippa, M.; Panza, C.; Ferrari, F.; Frassoldati, R.; Kuhn, P.; Balduzzi, S.; D’Amico, R. Systematic review of maternal voice interventions demonstrates increased stability in preterm infants. Acta Paediatr. 2017, 106, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Monson, B.B.; Rock, J.; Cull, M.; Soloveychik, V. Neonatal intensive care unit incubators reduce language and noise levels more than the womb. J. Perinatol. 2020, 40, 600–606. [Google Scholar] [CrossRef]
- Blennow, G.; Svenningsen, N.W.; Almquist, B. Noise levels in infant incubators (adverse effects?). Pediatrics 1974, 53, 29–32. [Google Scholar]
- Fernandez Zacarias, F.; Beira Jimenez, J.L.; Bustillo Velazquez-Gaztelu, P.J.; Hernandez Molina, R.; Lubian Lopez, S. Noise level in neonatal incubators: A comparative study of three models. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 150–154. [Google Scholar] [CrossRef]
- Parra, J.; de Suremain, A.; Berne Audeoud, F.; Ego, A.; Debillon, T. Sound levels in a neonatal intensive care unit significantly exceeded recommendations, especially inside incubators. Acta Paediatr. 2017, 106, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Lindvall, T.; Schwela, D.H. Guidelines for Community Noise; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Noise: A hazard for the fetus and newborn. American Academy of Pediatrics. Committee on Environmental Health. Pediatrics 1997, 100, 724–727. [Google Scholar]
- Graven, S.N. Sound and the developing infant in the NICU: Conclusions and recommendations for care. J. Perinatol. 2000, 20, S88–S93. [Google Scholar] [CrossRef]
- White, R.D. Recommended standards for the newborn ICU. J. Perinatol. 2007, 27, S4–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.D.; Consensus Committee on Recommended Design Standards for Advanced Neonatal Care. Recommended standards for newborn ICU design, 9th edition. J. Perinatol. 2020, 40, 2–4. [Google Scholar] [CrossRef]
- Crandall, I.B.; MacKenzie, D. Analysis of the Energy Distribution in Speech. Physical. Rev. 1922, 19, 221–232. [Google Scholar] [CrossRef]
- Cox, R.V.; Neto, S.F.D.C.; Lamblin, C.; Sherif, M.H. ITU-T coders for wideband, superwideband, and fullband speech communication [Series Editorial]. IEEE Commun. Mag. 2009, 47, 106–109. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Q.J.; Narayanan, S.S. Effect of bandwidth extension to telephone speech recognition in cochlear implant users. J. Acoust. Soc. Am. 2009, 125, EL77–EL83. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, H. Physical measurements of audition and their bearing on the theory of hearing. Bell Syst. Tech. J. 1923, 2, 145–180. [Google Scholar] [CrossRef]
- Allen, J.B. Harvey Fletcher’s role in the creation of communication acoustics. J. Acoust. Soc. Am. 1996, 99, 1825–1839. [Google Scholar] [CrossRef] [Green Version]
- French, N.R.; Steinberg, J.C. Factors Governing the Intelligibility of Speech Sounds. J. Acoust. Soc. Am. 1947, 19, 90–119. [Google Scholar] [CrossRef]
- Liao, J.; Liu, G.; Xie, N.; Wang, S.; Wu, T.; Lin, Y.; Hu, R.; He, H.G. Mothers’ voices and white noise on premature infants’ physiological reactions in a neonatal intensive care unit: A multi-arm randomized controlled trial. Int. J. Nurs. Stud. 2021, 119, 103934. [Google Scholar] [CrossRef]
- Gadeke, R.; Doring, B.; Keller, F.; Vogel, A. The noise level in a childrens hospital and the wake-up threshold in infants. Acta Paediatr. Scand. 1969, 58, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.; Lahav, A. Ototoxicity in preterm infants: Effects of genetics, aminoglycosides, and loud environmental noise. J. Perinatol. 2013, 33, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Bellieni, C.V.; Buonocore, G.; Pinto, I.; Stacchini, N.; Cordelli, D.M.; Bagnoli, F. Use of sound-absorbing panel to reduce noisy incubator reverberating effects. Biol. Neonate 2003, 84, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Abou Turk, C.; Williams, A.L.; Lasky, R.E. A randomized clinical trial evaluating silicone earplugs for very low birth weight newborns in intensive care. J. Perinatol. 2009, 29, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freudenthal, A.; van Stuijvenberg, M.; van Goudoever, J.B. A quiet NICU for improved infants’ health, development and well-being: A systems approach to reducing noise and auditory alarms. Cogn. Technol. Work 2012, 15, 329–345. [Google Scholar] [CrossRef] [Green Version]
- Casey, L.; Fucile, S.; Flavin, M.; Dow, K. A two-pronged approach to reduce noise levels in the neonatal intensive care unit. Early Hum. Dev. 2020, 146, 105073. [Google Scholar] [CrossRef]
- Busch-Vishniac, I.J.; West, J.E.; Barnhill, C.; Hunter, T.; Orellana, D.; Chivukula, R. Noise levels in Johns Hopkins Hospital. J. Acoust. Soc. Am. 2005, 118, 3629–3645. [Google Scholar] [CrossRef] [Green Version]
- Darcy, A.E.; Hancock, L.E.; Ware, E.J. A descriptive study of noise in the neonatal intensive care unit: Ambient levels and perceptions of contributing factors. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal. Nurses 2008, 8, S16–S26. [Google Scholar] [CrossRef]
- Lasky, R.E.; Williams, A.L. Noise and light exposures for extremely low birth weight newborns during their stay in the neonatal intensive care unit. Pediatrics 2009, 123, 540–546. [Google Scholar] [CrossRef]
- Berg, A.L. Monitoring Noise Levels in a Tertiary Neonatal. Intensive Care Unit. Contemp. Issues Commun. Sci. Disord. 2010, 37, 69–72. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Dee, A.; Philip, R.K. Evidence-based design for neonatal units: A systematic review. Matern Health Neonatol. Perinatol. 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Veenendaal, N.R.; van Kempen, A.; Franck, L.S.; O’Brien, K.; Limpens, J.; van der Lee, J.H.; van Goudoever, J.B.; van der Schoor, S.R.D. Hospitalising preterm infants in single family rooms versus open bay units: A systematic review and meta-analysis of impact on parents. EClinicalMedicine 2020, 23, 100388. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.F. Comparing sound measurements in the single-family room with open-unit design neonatal intensive care unit: The impact of equipment noise. J. Perinatol. 2012, 32, 368–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Enk, R.A.; Steinberg, F. Comparison of private room with multiple-bed ward neonatal intensive care unit. HERD 2011, 5, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chen, C.H.; Wu, C.C.; Huang, H.J.; Wang, T.M.; Hsu, C.C. The influence of neonatal intensive care unit design on sound level. Pediatr. Neonatol 2009, 50, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.C.; Akram Khan, M.; Munson, D.P.; Reid, E.J.; Helseth, C.C.; Buggy, J. The impact of architectural design upon the environmental sound and light exposure of neonates who require intensive care: An evaluation of the Boekelheide Neonatal. Intensive Care Nursery. J. Perinatol. 2007, 27, S20–S28. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.C.; Helseth, C.C.; Thompson, P.A.; Pottala, J.V.; Khan, M.A.; Munson, D.P. A Comprehensive Comparison of Open-Bay and Single-Family-Room Neonatal. Intensive Care Units at Sanford Children’s Hospital. HERD 2012, 5, 23–39. [Google Scholar] [CrossRef]
- Stevens, D.; Thompson, P.; Helseth, C.; Pottala, J. Mounting evidence favoring single-family room neonatal intensive care. J. Neonatal. Perinat. Med. 2015, 8, 177–178. [Google Scholar] [CrossRef] [Green Version]
- Surenthiran, S.S.; Wilbraham, K.; May, J.; Chant, T.; Emmerson, A.J.; Newton, V.E. Noise levels within the ear and post-nasal space in neonates in intensive care. Arch. Dis. Child. Fetal. Neonatal. Ed. 2003, 88, F315–F318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoehn, T.; Busch, A.; Krause, M.F. Comparison of noise levels caused by four different neonatal high-frequency ventilators. Intensive Care Med. 2000, 26, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kazemizadeh Gol, M.A.; Black, A.; Sidman, J. Bone conduction noise exposure via ventilators in the neonatal intensive care unit. Laryngoscope 2015, 125, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.; Augereau, T.; Johnsrude, I.S. Neural Responses and Perceptual Sensitivity to Sound Depend on Sound-Level Statistics. Sci. Rep. 2020, 10, 9571. [Google Scholar] [CrossRef]
- Job, R.F.; Hatfield, J.; Carter, N.L.; Peploe, P.; Taylor, R.; Morrell, S. General scales of community reaction to noise (dissatisfaction and perceived affectedness) are more reliable than scales of annoyance. J. Acoust. Soc. Am. 2001, 110, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, I.P. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex; Oxford Univ. Press: Oxford, UK, 1927; pp. xv, 430-xv, 430. [Google Scholar]
- Lim, N. Cultural differences in emotion: Differences in emotional arousal level between the East and the West. Integr. Med. Res. 2016, 5, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Kliuchko, M.; Heinonen-Guzejev, M.; Vuust, P.; Tervaniemi, M.; Brattico, E. A window into the brain mechanisms associated with noise sensitivity. Sci. Rep. 2016, 6, 39236. [Google Scholar] [CrossRef] [PubMed]
- Heinonen-Guzejev, M.; Vuorinen, H.S.; Mussalo-Rauhamaa, H.; Heikkila, K.; Koskenvuo, M.; Kaprio, J. Genetic component of noise sensitivity. Twin Res. Hum. Genet. 2005, 8, 245–249. [Google Scholar] [CrossRef]
- Brezmes-Raposo, M.; Bermudez, L.; Dominguez, C.; Fernandez, C.; Franco, A.; Villa, C.; Sanz, I.; Pino-Vazquez, A. P0503/#2118: Caring for the invisible. how to humanize clinical care in a pediatric and neonatal intensive care unit. Pediatric. Crit. Care Med. 2021, 22, 253. [Google Scholar]
- Wielek, T.; Del Giudice, R.; Lang, A.; Wislowska, M.; Ott, P.; Schabus, M. On the development of sleep states in the first weeks of life. PLoS ONE 2019, 14, e0224521. [Google Scholar] [CrossRef] [PubMed]
- Saliba, S.; Esseily, R.; Filippa, M.; Gratier, M.; Grandjean, D. Changes in the vocal qualities of mothers and fathers are related to preterm infant’s behavioural states. Acta Paediatr. 2020, 109, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Shinya, Y.; Kawai, M.; Niwa, F.; Imafuku, M.; Myowa, M. Fundamental Frequency Variation of Neonatal. Spontaneous Crying Predicts Language Acquisition in Preterm and Term Infants. Front. Psychol. 2017, 8, 2195. [Google Scholar] [CrossRef] [Green Version]
- McDermott, J.H.; Schultz, A.F.; Undurraga, E.A.; Godoy, R.A. Indifference to dissonance in native Amazonians reveals cultural variation in music perception. Nature 2016, 535, 547–550. [Google Scholar] [CrossRef]
- Olejnik, B.K. Inadvertent Noise in Neonatal. Intensive Care Unit and its Impact on Prematurely Born Infants. Biomed. J. Sci. Tech. Res. 2018, 11. [Google Scholar] [CrossRef]
- Bertsch, M.; Reuter, C.; Czedik-Eysenberg, I.; Berger, A.; Olischar, M.; Bartha-Doering, L.; Giordano, V. The “Sound of Silence” in a Neonatal. Intensive Care Unit-Listening to Speech and Music Inside an Incubator. Front. Psychol. 2020, 11, 1055. [Google Scholar] [CrossRef]
- Meyer, J.; Dentel, L.; Meunier, F. Speech recognition in natural background noise. PLoS ONE 2013, 8, e79279. [Google Scholar] [CrossRef] [Green Version]
- International Electrotechnical Commission. IEC 60268-16:2020 Sound System Equipment—Part 16: Objective Rating of Speech Intelligibility by Speech Transmission Index, 5th ed.; International Electrotechnical Commission: Geneva, Switzerland, 2020. [Google Scholar]
- Kuhl, P.K. Early language acquisition: Cracking the speech code. Nat. Rev. Neurosci. 2004, 5, 831–843. [Google Scholar] [CrossRef]
- Trehub, S.E.; Bull, D.; Schneider, B.A. Infants’ detection of speech in noise. J. Speech Hear. Res. 1981, 24, 202–206. [Google Scholar] [CrossRef]
- Spinelli, M.; Fasolo, M.; Mesman, J. Does prosody make the difference? A meta-analysis on relations between prosodic aspects of infant-directed speech and infant outcomes. Dev. Rev. 2017, 44, 1–18. [Google Scholar] [CrossRef]
- Aita, M.; Robins, S.; Charbonneau, L.; Doray-Demers, P.; Feeley, N. Comparing light and noise levels before and after a NICU change of design. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Ramesh, A.; Denzil, S.B.; Linda, R.; Josephine, P.K.; Nagapoornima, M.; Suman Rao, P.N.; Swarna Rekha, A. Maintaining reduced noise levels in a resource-constrained neonatal intensive care unit by operant conditioning. Indian Pediatr. 2013, 50, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ruben, R.J. The Ontogeny of Human Hearing. Acta Oto-Laryngol. 1992, 112, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, D.; Duong, K.M.; Gilmour, I.J.; Davies, M.W. NeoSTRESS: Study of Transfer and Retrieval Environmental Stressors upon neonates via a smartphone application—Sound. J. Paediatr. Child. Health 2020, 56, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Capriolo, C.; Viscardi, R.M.; Broderick, K.A.; Nassebeh, S.; Kochan, M.; Solanki, N.S.; Leung, J.C. Assessment of Neonatal. Intensive Care Unit Sound Exposure Using a Smartphone Application. Am. J. Perinatol. 2020. [Google Scholar] [CrossRef]
- Haslbeck, F.B.; Bassler, D. Music From the Very Beginning-A Neuroscience-Based Framework for Music as Therapy for Preterm Infants and Their Parents. Front. Behav. Neurosci. 2018, 12, 112. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restin, T.; Gaspar, M.; Bassler, D.; Kurtcuoglu, V.; Scholkmann, F.; Haslbeck, F.B. Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves. Children 2021, 8, 704. https://doi.org/10.3390/children8080704
Restin T, Gaspar M, Bassler D, Kurtcuoglu V, Scholkmann F, Haslbeck FB. Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves. Children. 2021; 8(8):704. https://doi.org/10.3390/children8080704
Chicago/Turabian StyleRestin, Tanja, Mikael Gaspar, Dirk Bassler, Vartan Kurtcuoglu, Felix Scholkmann, and Friederike Barbara Haslbeck. 2021. "Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves" Children 8, no. 8: 704. https://doi.org/10.3390/children8080704
APA StyleRestin, T., Gaspar, M., Bassler, D., Kurtcuoglu, V., Scholkmann, F., & Haslbeck, F. B. (2021). Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves. Children, 8(8), 704. https://doi.org/10.3390/children8080704







