Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Fetal Instrumentation
2.2. Meconium Instillation, Asphyxia and Resuscitation
2.3. Primary and Secondary Outcomes
2.4. Data Collection and Analysis
3. Results
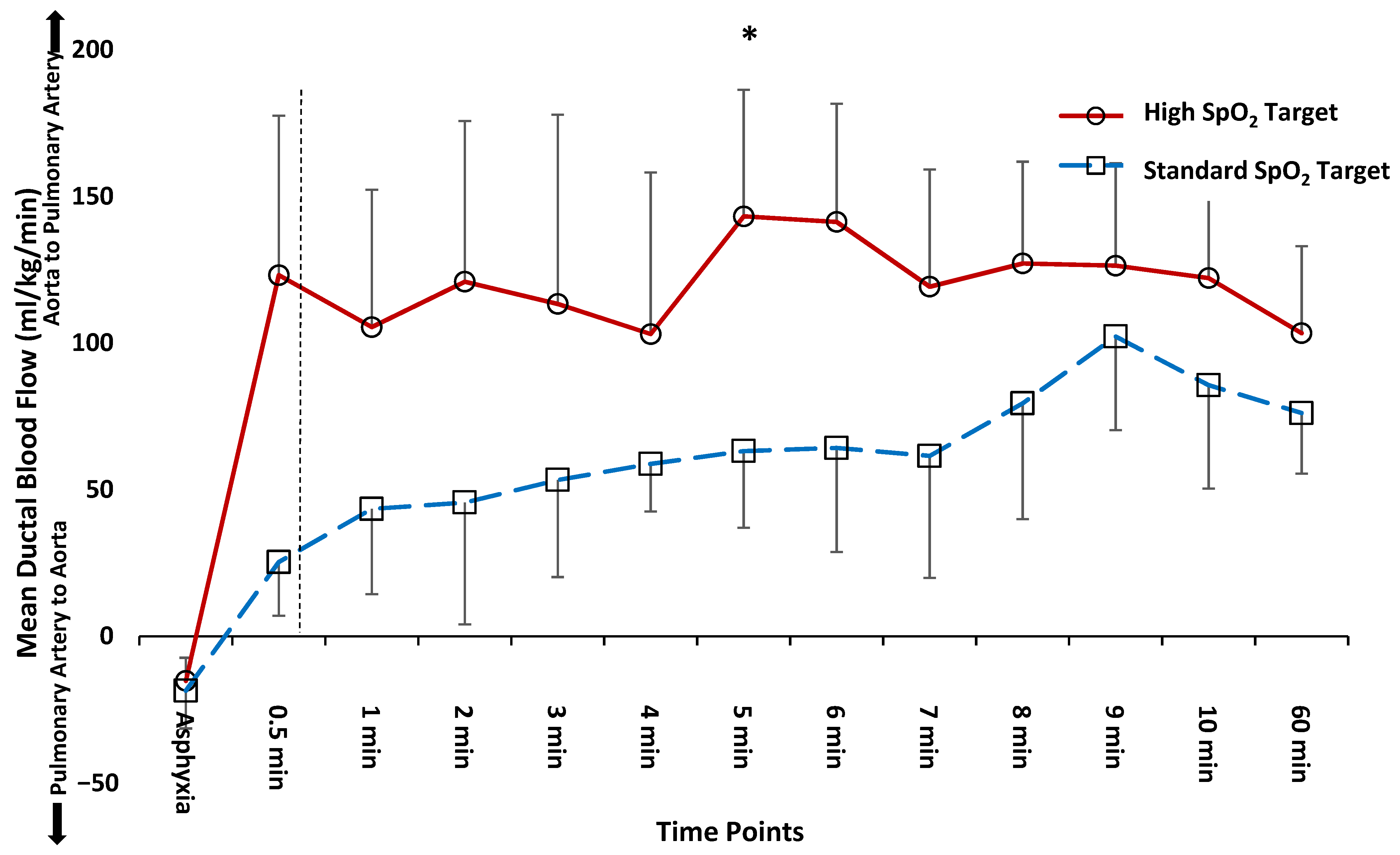
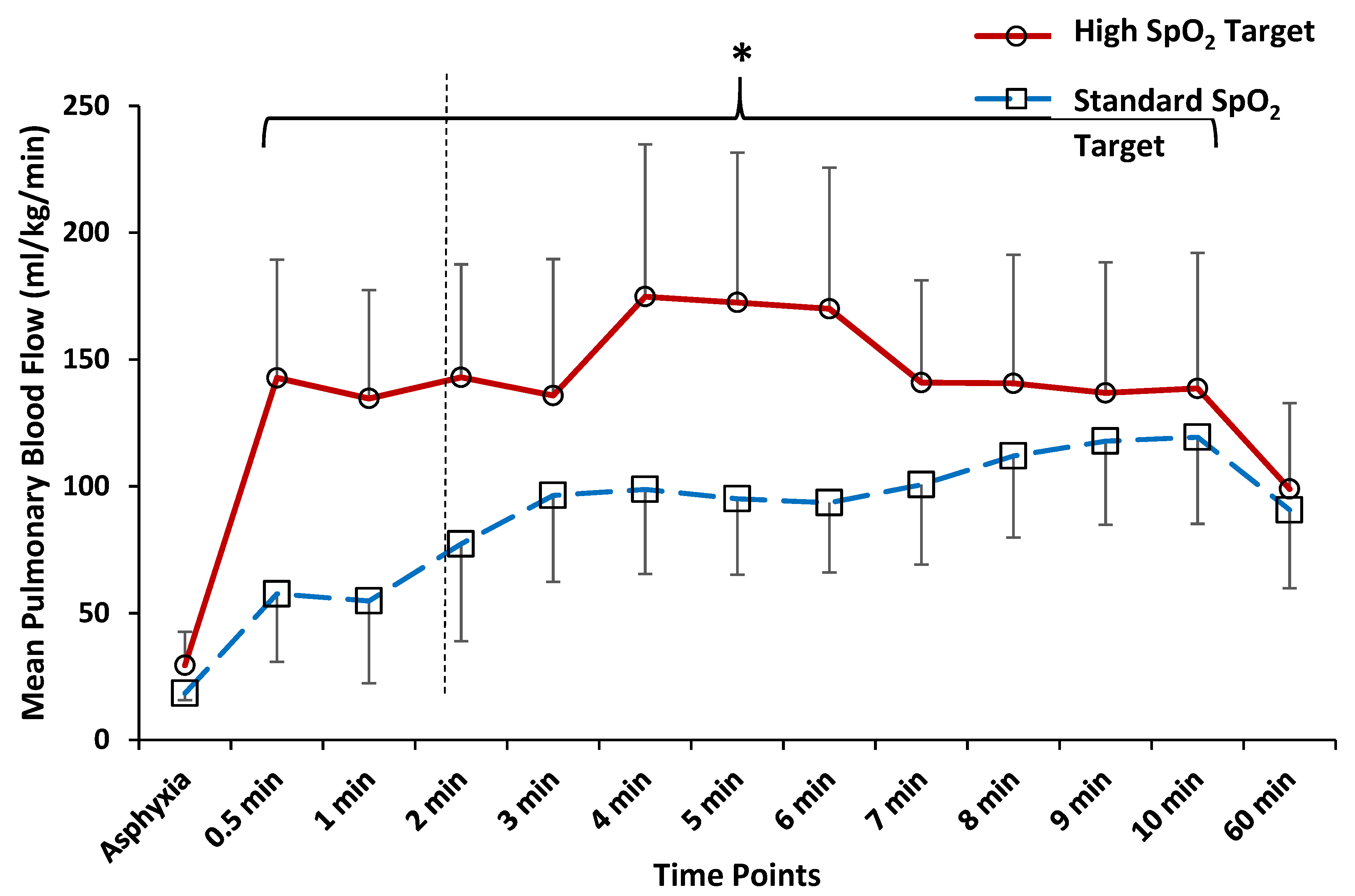
3.1. Time to Reversal of Shunt across the PDA
3.2. Comparison of Hemodynamics and Arterial Blood Gas Parameters at 5 and 10 min after Birth
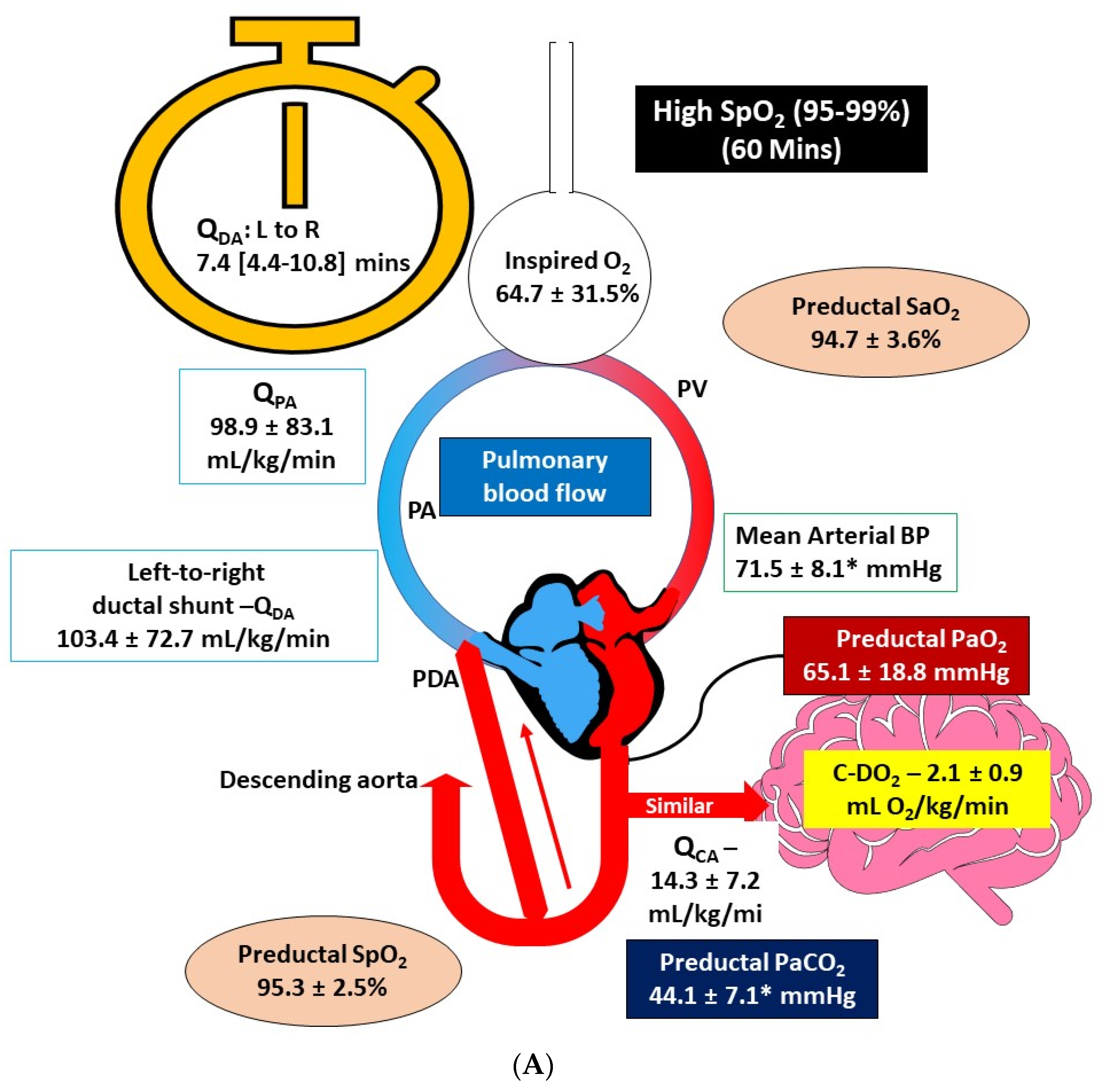
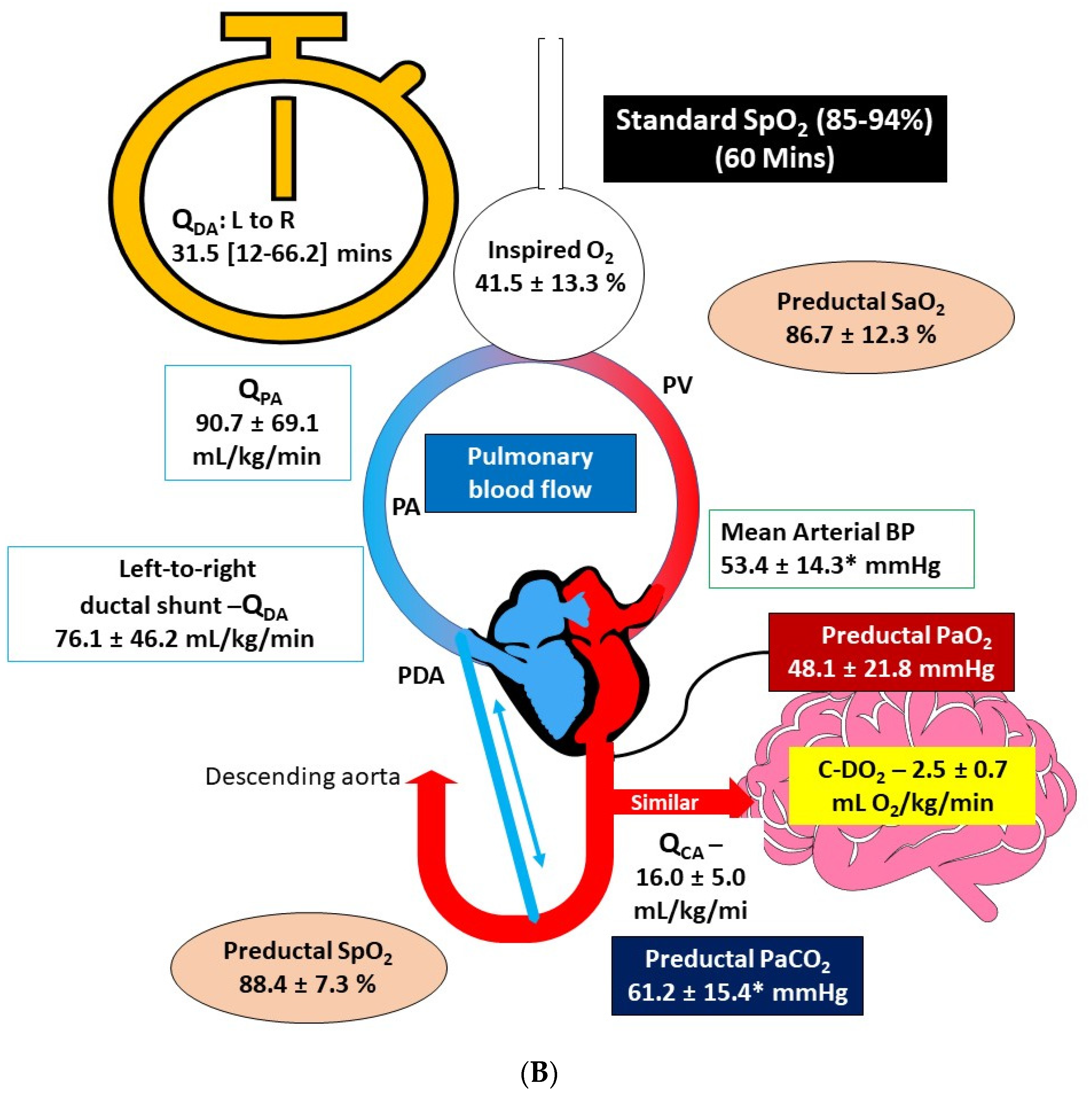
3.3. Comparison of Hemodynamics and Arterial Blood Gas Parameters at 60 min after Birth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Morin, F.C., 3rd; Egan, E.A.; Ferguson, W.; Lundgren, C.E. Development of pulmonary vascular response to oxygen. Am. J. Physiol. Heart Circ. Physiol. 1988, 254, H542–H546. [Google Scholar] [CrossRef] [PubMed]
- Teitel, D.F.; Iwamoto, H.S.; Rudolph, A.M. Changes in the Pulmonary Circulation during Birth-Related Events. Pediatr. Res. 1990, 27, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Chandrasekharan, P.K.; Swartz, D.D.; Mathew, B.; Nair, J.; Gugino, S.F.; Koenigsknecht, C.; Vali, P.; Lakshminrusimha, S. Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration. Pediatr. Res. 2016, 79, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Chandrasekharan, P.; Gugino, S.F.; Koenigsknecht, C.; Nielsen, L.; Wedgwood, S.; Mathew, B.; Nair, J.; Steinhorn, R.; Lakshminrusimha, S. Optimal Oxygen Targets in Term Lambs with Meconium Aspiration Syndrome and Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 63, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Smolich, J.J.; Kenna, K.R.; Mynard, J.P. Antenatal betamethasone augments early rise in pulmonary perfusion at birth in preterm lambs: Role of ductal shunting and right ventricular outflow distribution. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R716–R724. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Mathew, B.; Nair, J.; Gugino, S.F.; Koenigsknecht, C.; Rawat, M.; Nielsen, L.; Swartz, D.D. Tracheal suctioning improves gas exchange but not hemodynamics in asphyxiated lambs with meconium aspiration. Pediatr. Res. 2015, 77, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, D.; Chandrasekharan, P.K.; Gugino, S.F.; Koenigsknecht, C.; Helman, J.; Nair, J.; Mathew, B.; Rawat, M.; Vali, P.; Nielsen, L.; et al. Randomised trial of epinephrine dose and flush volume in term newborn lambs. Arch. Dis. Child. Fetal Neonatal Ed. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, D.; Vali, P.; Chen, P.; Lesneski, A.L.; Hardie, M.E.; Alhassen, Z.; Wedgwood, S.; Wyckoff, M.H.; Lakshminrusimha, S. Randomized trial of oxygen weaning strategies following chest compressions during neonatal resuscitation. Pediatr. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Weiner, G.M.; American Heart Association; Zaichkin, J. Textbook of Neonatal Resuscitation, 7th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2016. [Google Scholar]
- Walsh-Sukys, M.C.; Tyson, J.E.; Wright, L.L.; Bauer, C.R.; Korones, S.B.; Stevenson, D.K.; Verter, J.; Stoll, B.J.; Lemons, J.A.; Papile, L.A.; et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics 2000, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nakwan, N.; Chaiwiriyawong, P. An international survey on persistent pulmonary hypertension of the newborn: A need for an evidence-based management. J. Neonatal-Perinat. Med. 2016, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Jassar, R.; Shaffer, T.H. Management of Supplemental Oxygen for Infants with Persistent Pulmonary Hypertension of Newborn: A Survey. Am. J. Perinatol. 2017, 34, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.S.; Chalak, L.F.; DuPont, T.L.; Rollins, N.K.; Brion, L.P.; Wyckoff, M.H. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA 2018, 319, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiological interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E.; Peabody, S.S.; Davis, J.M.; Slayter, M.V.; Bent, R.C.; Merritt, T.A. Surfactant therapy and high-frequency jet ventilation in the management of a piglet model of the meconium aspiration syndrome. Pediatr. Res. 1994, 36, 494–500. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Parameter | Standard SpO2 Target (85–94%, n = 6) | High SpO2 Target (95–99%, n = 6) |
|---|---|---|
| Weight (kg) | 3.4 ± 0.8 | 3.3 ± 0.4 |
| Gestational Age (days) | 139.7 ± 0.7 | 139.3 ± 0.8 |
| Parameters at Fetal Baseline | ||
| Hemoglobin, g/dL | 13.88 ± 2.47 | 11.93 ± 1.52 |
| pH | 7.18 ± 0.08 | 7.19 ± 0.06 |
| PaCO2 (mm Hg) | 74.73 ± 12.58 | 64.95 ± 5.17 |
| PaO2 (mm Hg) | 22.08 ± 6.22 | 28.38 ± 4.51 |
| Cerebral Oxygen Delivery (mL/kg/min) | 4.26 ± 2.17 | 2.40 ± 0.64 |
| Lactate (mmol/L) | 2.20 ± 0.53 | 2.37 ± 0.96 |
| Heart Rate (bpm) | 155.17 ± 21.95 | 164.23 ± 21.50 |
| Mean Arterial Blood Pressure (mm Hg) | 55.52 ± 4.42 | 61.11 ± 3.8 |
| Mean Ductal Blood Flow (mL/kg/min) | −125.89 ± 64.77 | −84.33 ± 36.09 |
| Mean Pulmonary Artery Blood Flow (mL/kg/min) | 25.21 ± 8.25 | 49.39 ± 23.59 |
| Duration of Asphyxia (min) | 13.43 ± 0.63 | 15.32 ± 1.82 |
| Parameters at End of Asphyxia | ||
| Mean Carotid Artery Blood Flow (mL/kg/min) | 24.0 (5.5) | 21.2 (4.4) |
| Mean Ductal Blood Flow (mL/kg/min) | −18.3 (6.9) | −15 (7.7) |
| Mean Pulmonary Artery Blood Flow (mL/kg/min) | 18.3 (2.7) | 29.4 (13.2) |
| Parameter | Standard SpO2 Target (85–94%, n = 6) | High SpO2 Target (95–99%, n = 6) |
|---|---|---|
| Heart Rate (bpm) | 182.12 ± 12.14 | 170.78 ± 13.08 |
| Mean Arterial Blood Pressure (mm Hg) | 52.91 ± 18.79 | 65.38 ± 8.81 |
| Mean Carotid Flow (QCA, mL/kg/min) | 34.10 ± 14.84 | 20.22 ± 8.47 |
| Parameter | Standard SpO2 Target (85–94%, n = 6) | High SpO2 Target (95–99%, n = 6) |
|---|---|---|
| Hemoglobin, g/dL | 13.18 ± 1.95 | 11.9 ± 1.40 |
| pH | 7.05 ± 0.24 | 7.11 ± 0.19 |
| PaCO2 (mm Hg) | 79.48 ± 48.89 | 64.5 ± 42.89 |
| PaO2 (mm Hg) | 53.42 ± 22.97 | 60.13 ± 2.55 |
| SaO2 (%) | 89.7 ± 4.32 | 93.9 ± 3.96 |
| SpO2 (%) | 77.75 ± 30.61 | 91.33 ± 6.51 |
| CaO2 (mL O2/dL) | 15.98 ± 2.14 | 15.14 ± 1.62 |
| Cerebral Oxygen Delivery (mL/kg/min) | 4.78 ± 2.23 | 3.69 ± 0.47 |
| Lactate (mmol/L) | 6.02 ± 1.97 | 5.90 ± 1.75 |
| Heart Rate (bpm) | 176.0 ± 13.22 | 162.55 ± 31.18 |
| Mean Arterial Blood Pressure (mmHg) | 59.34 ± 9.497 | 65.67 ± 8.98 |
| Mean Carotid Flow (QCA, mL/kg/min) | 29.17 ± 12.31 | 20.44 ± 7.32 |
| Inspired O2 (%) at 10 min | 40.75 ± 39.5 | 30.67 ± 16.74 |
| Parameter | Standard SpO2 Target (85–94%, n = 6) | High SpO2 Target (95–99%, n = 6) |
|---|---|---|
| Hemoglobin, g/dL | 12.32 ± 3.17 | 11.85 ± 1.08 |
| pH | 7.12 ± 0.11 | 7.25 ± 0.09 ǂ |
| PaCO2 (mmHg) | 61.23 ± 15.42 | 44.08 ± 7.07 ǂ |
| PaO2 (mmHg) | 48.14 ± 21.76 | 65.08 ± 18.77 |
| SaO2 (%) | 86.66 ± 12.29 | 94.68 ± 3.55 |
| SpO2 (%) | 88.4 ± 7.3 | 95.33 ± 2.50 |
| CaO2 (mlO2/dL) | 14.96 ± 4.22 | 15.23 ± 1.44 |
| Cerebral Oxygen Delivery (ml/kg/min) | 2.54 ± 0.68 | 2.12 ± 0.9 |
| Lactate (mmol/L) | 4.25 ± 1.94 | 4.07 ± 1.75 |
| Heart Rate (bpm) | 167.76 ± 32.46 | 154.56 ± 9.94 |
| Mean Arterial Blood Pressure (mmHg) | 53.41 ± 14.30 | 71.51 ± 8.14 ǂ |
| Mean Carotid Flow (QCA-mL/kg/min) | 16.01 ± 4.96 | 14.28 ± 7.21 |
| Inspired O2 (%) at 60 min | 41.5 ± 13.25 | 64.66 ± 31.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesneski, A.L.; Vali, P.; Hardie, M.E.; Lakshminrusimha, S.; Sankaran, D. Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension. Children 2021, 8, 594. https://doi.org/10.3390/children8070594
Lesneski AL, Vali P, Hardie ME, Lakshminrusimha S, Sankaran D. Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension. Children. 2021; 8(7):594. https://doi.org/10.3390/children8070594
Chicago/Turabian StyleLesneski, Amy L., Payam Vali, Morgan E. Hardie, Satyan Lakshminrusimha, and Deepika Sankaran. 2021. "Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension" Children 8, no. 7: 594. https://doi.org/10.3390/children8070594
APA StyleLesneski, A. L., Vali, P., Hardie, M. E., Lakshminrusimha, S., & Sankaran, D. (2021). Randomized Trial of Oxygen Saturation Targets during and after Resuscitation and Reversal of Ductal Flow in an Ovine Model of Meconium Aspiration and Pulmonary Hypertension. Children, 8(7), 594. https://doi.org/10.3390/children8070594






