Prediction Model for Diagnosis of Kawasaki Disease Using iTRAQ-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
2.2. ITRAQ Gel-Free Proteomics
2.3. ELISA Validation for Selected Candidate Proteins
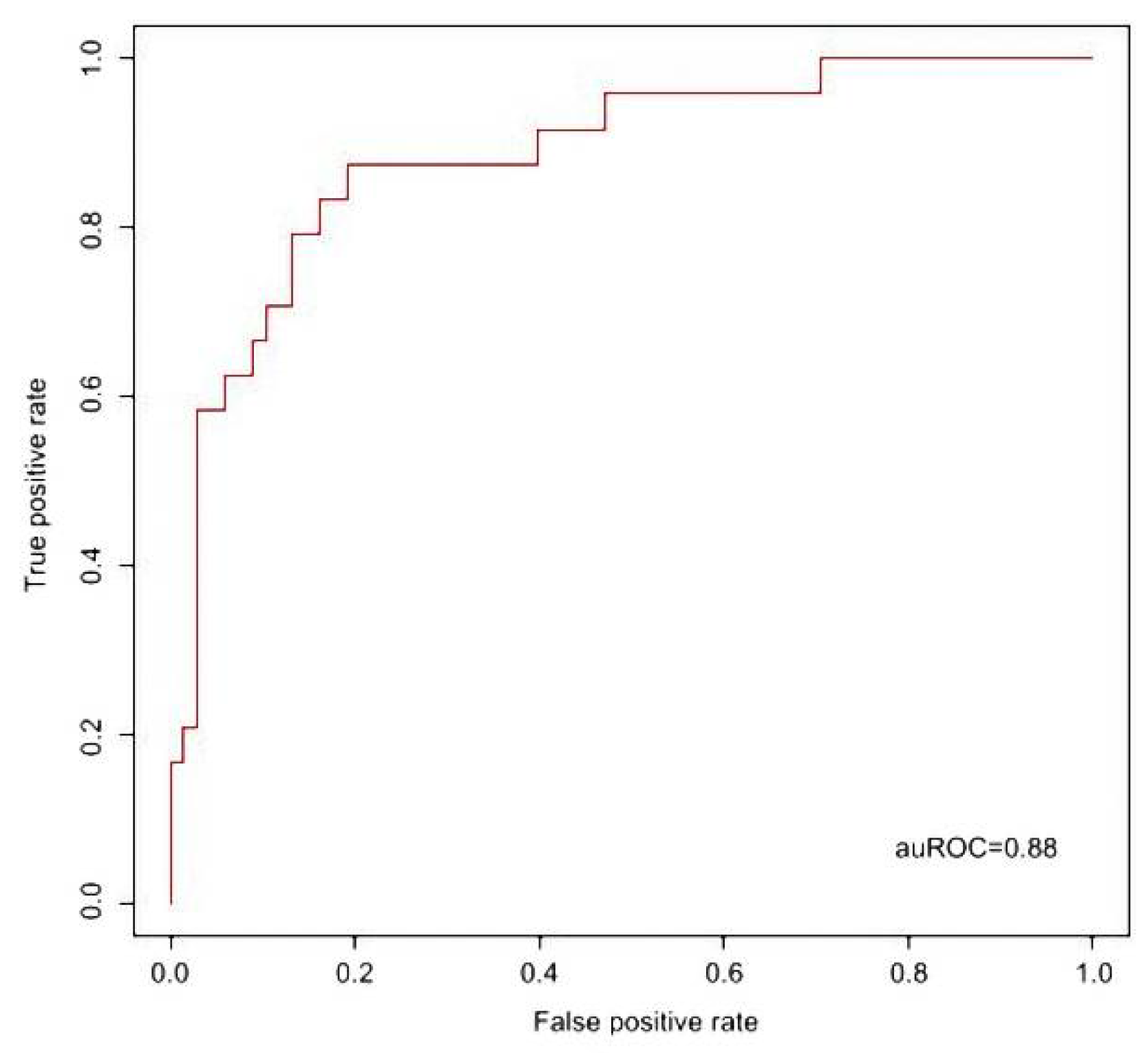
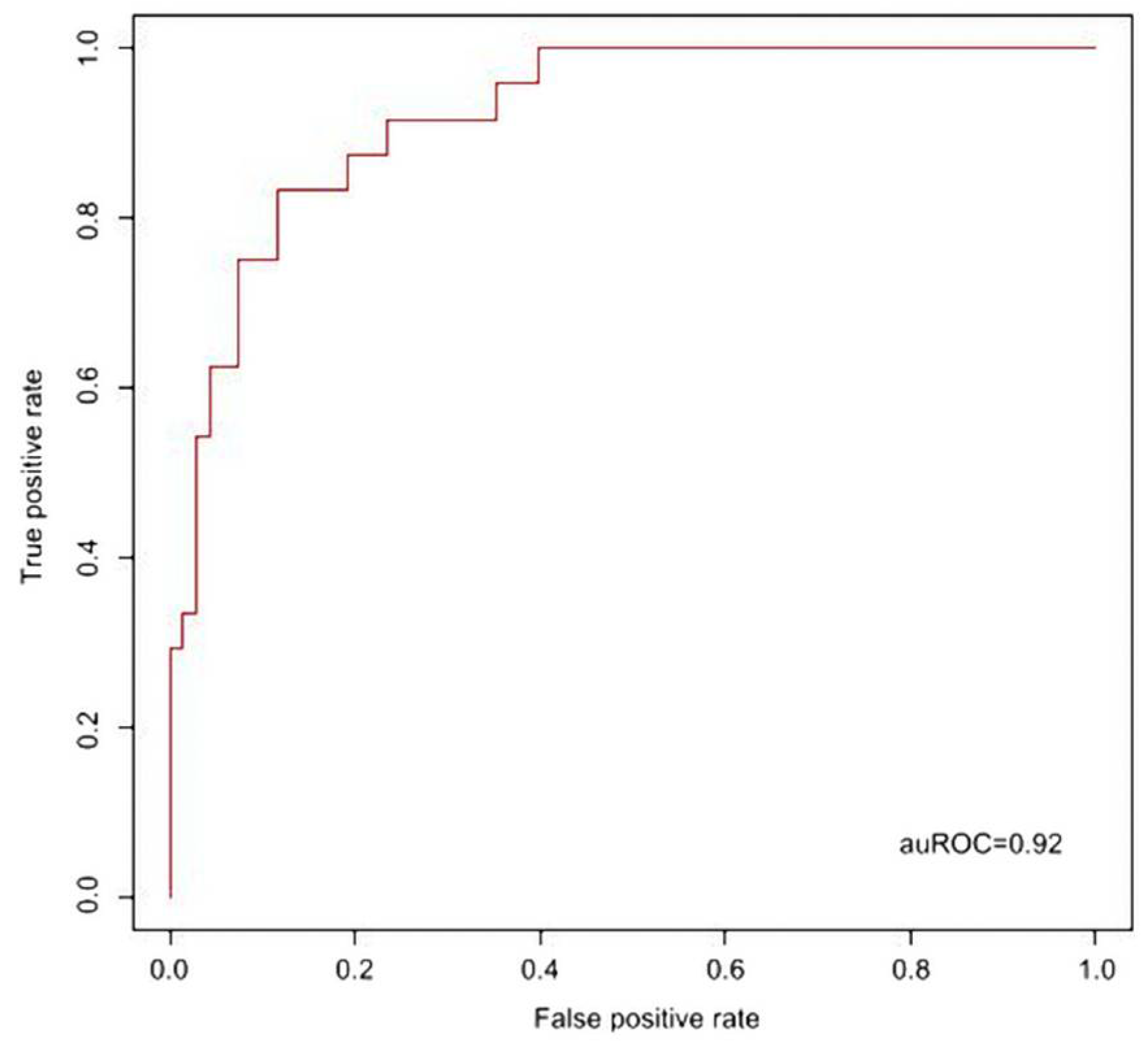
2.4. Support Vector Machine (SVM) Alignment for Prediction Model of KD Diagnosis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hsieh, K.-S.; Weng, K.-P.; Lin, C.-C.; Huang, T.-C.; Lee, C.-L.; Huang, S.-M. Treatment of acute Kawasaki disease: Aspirin’s role in the febrile stage revisited. Pediatrics 2004, 114, e689–e693. [Google Scholar] [CrossRef] [Green Version]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Tremoulet, A.H.; Best, B.M.; Song, S.; Wang, S.; Corinaldesi, E.; Eichenfield, J.R.; Martin, D.D.; Newburger, J.W.; Burns, J.C. Resistance to Intravenous Immunoglobulin in Children with Kawasaki Disease. J. Pediatr. 2008, 153, 117–121.e3. [Google Scholar] [CrossRef] [Green Version]
- Maury, C.P.; Salo, E.; Pelkonen, P. Circulating Interleukin-1β in Patients with Kawasaki Disease. N. Engl. J. Med. 1988, 319, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Furukawa, S.; Yabuta, K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin. Immunol. Immunopathol. 1990, 56, 29–36. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, C.C.; Hwang, B.; Chiang, B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J. Pediatr. 1992, 121, 924–926. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.K.; Noh, G.W.; Lee, S.I.; Lee, K.Y. Increased serum interleukin-10 level in Kawasaki disease. Yonsei Med. J. 1996, 37, 125–130. [Google Scholar] [CrossRef]
- Hirao, J.; Hibi, S.; Andoh, T.; Ichimura, T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int. Arch. Allergy Immunol. 1997, 112, 152–156. [Google Scholar] [CrossRef]
- Weng, K.-P.; Hsieh, K.-S.; Huang, S.-H.; Ou, S.-F.; Lai, T.-J.; Tang, C.-W.; Lin, C.-C.; Ho, T.-Y.; Liou, H.-H.; Ger, L.-P. Interleukin-18 and coronary artery lesions in patients with Kawasaki disease. J. Chin. Med. Assoc. 2013, 76, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Furuno, K.; Takada, H.; Yamamoto, K.; Ikeda, K.; Ohno, T.; Khajoee, V.; Mizuno, Y.; Hara, T. Tissue Inhibitor of Metalloproteinase 2 and Coronary Artery Lesions in Kawasaki Disease. J. Pediatr. 2007, 151, 155–160.e1. [Google Scholar] [CrossRef]
- Gavin, P.J.; Crawford, S.E.; Shulman, S.T.; Garcia, F.L.; Rowley, A. Systemic Arterial Expression of Matrix Metalloproteinases 2 and 9 in Acute Kawasaki Disease. Arter. Thromb. Vasc. Biol. 2003, 23, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Sakata, K.; Hamaoka, K.; Ozawa, S.; Niboshi, A.; Yahata, T.; Fujii, M.; Hamaoka, A.; Toiyama, K.; Nishida, M.; Itoi, T. Matrix Metalloproteinase-9 in Vascular Lesions and Endothelial Regulation in Kawasaki Disease. Circ. J. 2010, 74, 1670–1675. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, S.; Tokutomi, T.; Kawase, H.; Nakatani, K.; Tsujimoto, H.; Kawamura, Y.; Sekine, I. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin. Exp. Immunol. 2001, 125, 340–344. [Google Scholar] [CrossRef]
- Terai, M.; Yasukawa, K.; Narumoto, S.; Tateno, S.; Oana, S.; Kohno, Y. Vascular endothelial growth factor in acute Kawasaki disease. Am. J. Cardiol. 1999, 83, 337–339. [Google Scholar] [CrossRef]
- Ohno, T.; Yuge, T.; Kariyazono, H.; Igarashi, H.; Joh-O, K.; Kinugawa, N.; Kusuhara, K.; Hara, T. Serum hepatocyte growth factor combined with vascular endothelial growth factor as a predictive indicator for the occurrence of coronary artery lesions in Kawasaki disease. Eur. J. Nucl. Med. Mol. Imaging 2001, 161, 105–111. [Google Scholar] [CrossRef]
- Breunis, W.B.; Davila, S.; Shimizu, C.; Oharaseki, T.; Takahashi, K.; Van Houdt, M.; Khor, C.C.; Wright, V.J.; Levin, M.; Burns, J.C.; et al. Disruption of vascular homeostasis in patients with Kawasaki disease: Involvement of vascular endothelial growth factor and angiopoietins. Arthritis Rheum. 2011, 64, 306–315. [Google Scholar] [CrossRef]
- Porritt, R.A.; Markman, J.L.; Maruyama, D.; Kocaturk, B.; Chen, S.; Lehman, T.J.A.; Lee, Y.; Fishbein, M.C.; Noval Rivas, M.; Arditi, M. Interleukin-1 beta-mediated sex differences in Kawasaki disease vasculitis development and response to treatment. Arter. Thromb. Vasc. Biol. 2020, 40, 802–818. [Google Scholar] [CrossRef]
- Kwon, J.E.; Roh, D.E.; Kim, Y.H. The impact of moderate-dose acetylsalicylic acid in the reduction of inflammatory cytokine and prevention of complication in acute phase of Kawasaki disease: The benefit of moderate-dose acetylsalicylic acid. Children 2020, 7, 185. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, H.-L.; Huang, W.-M.; Liu, C.-W.; Hua, L.; Liu, T.-C.; Mao, L.-J.; Xu, Y.-F.; Li, W.; Xia, S.-L.; et al. Monitoring of the serum proteome in Kawasaki disease patients before and after immunoglobulin therapy. Biochem. Biophys. Res. Commun. 2014, 447, 19–25. [Google Scholar] [CrossRef]
- Kimura, Y.; Yanagimachi, M.; Ino, Y.; Aketagawa, M.; Matsuo, M.; Okayama, A.; Shimizu, H.; Oba, K.; Morioka, I.; Imagawa, T.; et al. Identification of candidate diagnostic serum biomarkers for Kawasaki disease using proteomic analysis. Sci. Rep. 2017, 7, 43732. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Huang, Y.-H.; Chung, F.-H.; Chen, P.-C.; Sung, T.-C.; Chen, Y.-W.; Hsieh, K.-S.; Chen, C.-S.; Syu, G.-D. Antibody Profiling of Kawasaki Disease Using Escherichia coli Proteome Microarrays. Mol. Cell. Proteom. 2018, 17, 472–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.-F.; Chu, H.-J.; Xu, Y.-F.; Hua, L.; Wang, Z.-P.; Huang, P.; Jia, H.-L.; Zhang, L. Proteomics study of serum exosomes in Kawasaki disease patients with coronary artery aneurysms. Cardiol. J. 2019, 26, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagida, M.; Kawasaki, M.; Fujishiro, M.; Miura, M.; Ikeda, K.; Nozawa, K.; Kaneko, H.; Morimoto, S.; Takasaki, Y.; Ogawa, H.; et al. Serum Proteome Analysis in Patients with Rheumatoid Arthritis Receiving Therapy with Tocilizumab: An Anti-Interleukin-6 Receptor Antibody. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Q.; Zhang, R.; Wang, Y.; Niu, F.; Wang, W.; Sun, D.; Wang, A. ITRAQ-Based Proteomics Analysis of Acute Lung Injury Induced by Oleic Acid in Mice. Cell. Physiol. Biochem. 2017, 44, 1949–1964. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Hsieh, K.-S.; Guo, M.M.-H.; Weng, K.-P.; Ger, L.-P.; Chan, W.-C.; Li, S.-C. Next-generation sequencing identifies micro-RNA–based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 2016, 138, 1227–1230. [Google Scholar] [CrossRef] [Green Version]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Ko, T.-M.; Kuo, H.-C.; Chang, J.-S.; Chen, S.-P.; Liu, Y.-M.; Chen, H.-W.; Tsai, F.-J.; Lee, Y.-C.; Chen, C.-H.; Wu, J.-Y.; et al. CXCL10/IP-10 Is a Biomarker and Mediator for Kawasaki Disease. Circ. Res. 2015, 116, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-C.; Tsai, K.-W.; Huang, L.-H.; Weng, K.-P.; Chien, K.-J.; Lin, Y.; Tu, C.-Y.; Lin, P.-H. Serum proteins may facilitate the identification of Kawasaki disease and promote in vitro neutrophil infiltration. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Fu, S.; Gong, F.; Xie, C.; Zhu, W.; Wang, W.; Shen, H.; Tang, Y. S100A12 on Circulating Endothelial Cells Surface in Children With Kawasaki Disease. Pediatr. Res. 2010, 68, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Armaroli, G.; Verweyen, E.; Pretzer, C.; Kessel, K.; Hirono, K.; Ichida, F.; Okabe, M.; Cabral, D.A.; Foell, D.; Brown, K.; et al. Monocyte-Derived Interleukin-1β As the Driver of S100A12-Induced Sterile Inflammatory Activation of Human Coronary Artery Endothelial Cells: Implications for the Pathogenesis of Kawasaki Disease. Arthritis Rheumatol. 2019, 71, 792–804. [Google Scholar] [CrossRef]
- Lech, M.; Guess, J.; Duffner, J.; Oyamada, J.; Shimizu, C.; Hoshino, S.; Farutin, V.; Bulik, D.A.; Gutierrez, B.; Sarvaiya, H.; et al. Circulating Markers of Inflammation Persist in Children and Adults With Giant Aneurysms After Kawasaki Disease. Circ. Genom. Precis. Med. 2019, 12, e002433. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Lu, W. Defensins in innate immunity. Curr. Opin. Hematol. 2014, 21, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C. The Immune Functions of α 1 Acid Glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef] [PubMed]
| KD (n = 68) | FC (n = 24) | p | |
|---|---|---|---|
| Age (years) | 1.8 ± 1.2 | 2.3 ± 1.3 | NS |
| M/F | 36/32 | 10/24 | NS |
| Blood sampling time since fever onset (day) | 5.8 ± 0.8 | 5.7 ± 0.9 | NS |
| Fever duration ≥ 5 days (%) | 100 | 100 | NS |
| Nonpurulent conjunctivitis (%) | 96 | 42 | <0.001 |
| Fissured lips or strawberry tongues (%) | 91 | 37 | <0.001 |
| Polymorphous rash (%) | 88 | 33 | <0.001 |
| Cervical adenopathy (%) | 53 | 50 | NS |
| Extremity edema or erythema (%) | 79 | 12 | <0.001 |
| BCG site erythema (%) | 63 | 0 | <0.001 |
| KD (n = 68) | FC (n = 24) | p | |
|---|---|---|---|
| CRP (mg/dL) | 6.8 ± 5.4 | 2.9 ± 3.9 | <0.001 |
| GOT (U/L) | 66.7 ± 118.1 | 51.5 ± 14.5 | NS |
| GPT (U/L) | 81.7 ± 87.2 | 25.9 ± 11.9 | <0.001 |
| WBC (/mm3) | 11897.4 ± 4195.7 | 7952.8 ± 3394.2 | <0.001 |
| Hgb (g/dL) | 11.1 ± 1.1 | 11.9 ± 1.1 | <0.01 |
| platelet (×1000/mm3) | 352.5 ± 122.3 | 240.8 ± 100.6 | <0.001 |
| Segment (%) | 52.9 ± 17.0 | 39.3 ± 21.2 | <0.01 |
| KD (n = 68) | FC (n = 24) | p | |
|---|---|---|---|
| S100A8 (ng/mL) | 717.9 ± 154.3 | 413.2 ± 223.9 | <0.001 |
| S100A9 (ng/mL) | 119.8 ± 32.9 | 70.6 ± 43.8 | <0.001 |
| S100A12 (ng/mL) | 798.4 ± 603.9 | 337.5 ± 410.8 | <0.001 |
| ORM1 (ng/mL) | 5375.1 ± 1490.6 | 3047.7 ± 1137.6 | <0.001 |
| DEFA1 (μg/mL) | 2611.7 ± 2535.2 | 1193.9 ± 1344.4 | <0.001 |
| CRP | GOT | GPT | WBC | Platelet | Segment | Hgb | |
|---|---|---|---|---|---|---|---|
| S100A8 | 0.37 *** | 0.07 | 0.19 | 0.42 **** | 0.41 **** | 0.53 **** | −0.19 |
| S100A9 | 0.33 ** | 0.12 | 0.21 * | 0.44 **** | 0.41 **** | 0.55 **** | −0.02 |
| S100A12 | 0.19 | 0.24 * | 0.13 | 0.37 *** | 0.33 ** | 0.36 *** | −0.21 * |
| DEFA1 | 0.26 * | 0.19 | 0.24 * | 0.15 | 0.18 | 0.26 ** | −0.27 ** |
| ORM1 | 0.47 **** | 0.06 | 0.19 | 0.24 * | 0.25 * | 0.37 *** | −0.33 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, K.-P.; Li, S.-C.; Chien, K.-J.; Tsai, K.-W.; Kuo, H.-C.; Hsieh, K.-S.; Huang, S.-H. Prediction Model for Diagnosis of Kawasaki Disease Using iTRAQ-Based Analysis. Children 2021, 8, 576. https://doi.org/10.3390/children8070576
Weng K-P, Li S-C, Chien K-J, Tsai K-W, Kuo H-C, Hsieh K-S, Huang S-H. Prediction Model for Diagnosis of Kawasaki Disease Using iTRAQ-Based Analysis. Children. 2021; 8(7):576. https://doi.org/10.3390/children8070576
Chicago/Turabian StyleWeng, Ken-Pen, Sung-Chou Li, Kuang-Jen Chien, Kuo-Wang Tsai, Ho-Chang Kuo, Kai-Sheng Hsieh, and Shih-Hui Huang. 2021. "Prediction Model for Diagnosis of Kawasaki Disease Using iTRAQ-Based Analysis" Children 8, no. 7: 576. https://doi.org/10.3390/children8070576
APA StyleWeng, K.-P., Li, S.-C., Chien, K.-J., Tsai, K.-W., Kuo, H.-C., Hsieh, K.-S., & Huang, S.-H. (2021). Prediction Model for Diagnosis of Kawasaki Disease Using iTRAQ-Based Analysis. Children, 8(7), 576. https://doi.org/10.3390/children8070576







